Sizing Up the Benefits of Sterile Drug Manufacturing Techniques
Sterile manufacturing environments are open to many sources of potential contamination if not managed correctly: air filtration systems, materials transfer and, not to mention, operators — a fully gowned operator may create as many as 150,000 particles per minute, many of which are viable and are potential hazards during the manufacture of sterile drugs. The need to ensure the safe and sterile transfer of active pharmaceutical ingredients (APIs) and formulation ingredients during aseptic processing has driven the development of multiple techniques that can be employed in cleanroom environments to minimize the risks from contaminants.
APPROACHES TO ASEPTIC MANUFACTURING
Barrier technologies, such as isolators and RABs, are designed to provide micro-environments which have been seen to provide better levels of microbiological protection for a process than that of traditional open cleanroom environments. The aim is to separate the operator from the product and prevent harmful contamination spreading to critical operations in the drug production process.
Isolators
Isolators are an arrangement of physical barriers that provide an enclosed working space that is detached from the surrounding environment. This enables manipulation to be undertaken within the space from the outside the enclosure without compromising integrity.
These enclosures use a combination of techniques to provide and maintain the clean environment such as positively pressurized chambers maintained through closed loop control of the chamber pressure. HEPA filtered air, supplied to the chamber in a laminar flow to ensure particulate generation is suppressed and removed efficiently and integrated bio decontamination systems, to provide a six-log reduction to the chamber surfaces.
Due to the high-performance requirements for these enclosures, integrated pressure decay tests have become the norm during start up and prior to any bio decontamination phase, with the leak of the chamber being a key factor in the classification of the device. See ISO14644 on leak rates for separative devices.
RABS
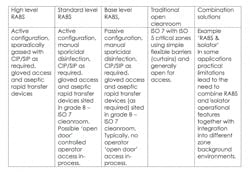
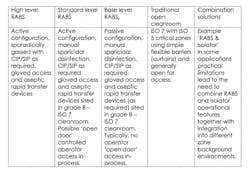
The RABS approach puts a physical barrier between operators and production areas, while still offering the flexibility to interact with the process outside a sealed enclosure. To allow a more limited barrier to be permissible, RABS must be set-up in high-class, generally ISO 7 cleanrooms.
There are two different types of RABS which are commonly used. The first are active RABs, which actively pull the air from outside the cleanroom environment, filtering and extracting it so it is completely isolated. The second type are standard passive RABS that utilize the cleanrooms (HVAC) system. Within these two formats, these are sub-categories which can be defined as follows:
RABS contribute distinct advantages by enabling operators to maintain a distance from the process, while allowing the enclosure to be opened if significant intervention is required.
Comparisons between isolators and RABS
In comparison to isolators, RABS can ensure faster start-up times and improve the ease of changeover. They can also bring increased operational flexibility and reduced validation expenditure. Although Isolators do offer the advantage of higher integrity chambers for a more robust closed solution.
Many pharmaceutical companies are finding that the use of aseptic SBV technology integrated to either the isolator or RABS for the transfer of material in or out of the enclosure compliments the sterility assurance required for the encloses.
SBV technology
The aseptic Spilt Butterfly Valve (SBV) provides a safe method of transferring product from one container, process vessel, Isolator or RABS to another while ensuring the sterility of the transfer is not compromised.
Fundamentally, the SBV consists of two halves, the active (Alpha) unit and the passive (Beta) unit. Each half consists of half of the ‘butterfly’ disc. The active unit is attached to the stationary process equipment such as a mixing vessel, while the passive unit is attached to the mobile container such as an intermediate bulk container (IBC) or flexible bag. When the two parts are brought together, the discs halves join to form a single disc, sealing any surfaces which may have been exposed to a compound during transfer. The two discs then operate as one disc and can be opened to allow transfer of product from one location to another.
The unique design of the aseptic SBV enables decontamination to take place in a closed environment. Once sealed, a gap is created between the discs and hydrogen peroxide gas is flushed through this enclosure to decontaminate the space. The process of validation is done in the same manner as that of an Isolator or RABS with the use of, chemical indicators to ensure full coverage of the enclosure is obtained and biological indicators to ensure a 6-log reduction has been achieved.
Adoption of aseptic SBV technology allows manufacturers to benefit from a closed handling method that not only achieves the required sterility assurance level (SAL) and reduces the requirement for manual intervention, but also offers the opportunity to reduce the resource associated with cleaning and validating large areas. The method minimizes cleaning requirements and, consequently, downtime, while also increasing flow and yield from product transfers.
Processing time varies between 4-30 minutes depending on the gassing system utilized. This is extremely fast when compared to a conventional airlock or Isolator which could be in the region of four to six hours. SBV can also contribute considerable cost savings in comparison to traditional approaches, being as much as three to five times cheaper than alternative methods. The aseptic SBV also makes it possible to downgrade the surrounding cleanroom environment because of the integrity of the approach.
EACH TECHNOLOGY HAS ITS PLACE
The selection of an appropriate aseptic transfer technology will come down to a variety of factors, including the unique requirements and possibilities within a manufacturing facility, as well as the type of products being processed. Isolators are easier to decontaminate, monitor and offer a high degree of sterility assurance, while RABS provide both increased operational flexibility and speed of changeover which appeals to many manufacturers, particularly contract manufacturers that need to be able to adapt to different customer products and manufacturing processes. Aseptic SBV technology not only complements and works in harmony with these solutions, but in some applications, can replace them, reducing the reliance on cleanroom environments while offering increased sterility assurance and improved ergonomics. Barrier systems are vital to ensure product quality and operator protection, and each of these technologies has its place. It is vital that a full evaluation of products and processes against the various systems takes place in an early project phase to ensure the right technology is selected.
Christian is the Global Product Manager for ChargePoint Technology for the aseptic range of products. Over the past 15 years, Christian has been creating innovative solutions for the pharmaceutical, biotech, cell therapy and fine chemical industries in the form of high containment and aseptic process solutions.