Few industries have as much to gain from data-driven decision making as pharmaceutical manufacturing. The disruptive forces impacting the industry — rising cost pressure, greater use of contract manufacturing, the introduction of personalized medicine, increasing packaging complexity, unrelenting quality control demands — cry out for the insights achievable through big data techniques.
Steering the industry through these disruptions will require not only rapid technical advancement, but also organizational and cultural adjustment. The industry’s culture is marked by a widespread, if largely unstated, belief that more information can be dangerous, as it invites more regulatory scrutiny and increased cost of compliance. This cultural legacy has become counterproductive and must be shed by companies seeking to thrive in the era of big data.
Pharmaceutical and life science manufacturers have traditionally been more reactive than proactive in technology adoption largely due to regulations and domain complexities. Success in life sciences has historically depended far more heavily on research and product innovation than on manufacturing prowess. But challenging market conditions are now pushing companies to innovate in using data visibility and analytics to improve manufacturing.
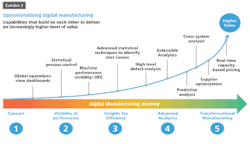
Most large pharmaceutical and life sciences companies have undertaken digital manufacturing initiatives. The majority are in early stages, doing proof of concept projects, but a growing minority — after demonstrating initial results — have begun rolling out those initiatives at scale.
WHAT IT TAKES TO SUCCEED IN A DIGITAL MANUFACTURING PROJECT
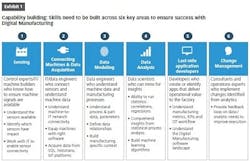
Pharma companies face similar challenges in digital transformation as their peers in other industries, and similar combinations of technical and organizational skills are needed for projects to succeed. Knowing what to look for in both sets of skills is important for selecting appropriate first-mover projects to gain proof points and momentum.
On the technical side, making the data available is the first step. At a foundational level, the key machines must be networked, and the data must be captured. Remote visualization of production data requires appropriate cloud and network technology, and companies need to be mindful of security while embracing new technology adoption. This provides a foundation of raw data to feed data models and sophisticated algorithms.
Companies also must navigate organizational challenges. If the top of a company is committed to digital transformation but plant leadership sees it as a threat, the project is unlikely to succeed. Similarly, an initiative at the plant level may struggle to reach full potential without the buy-in of corporate IT leadership. Also, an organization’s ability to realize the benefits of new technology adoption relies on change management, so there must be committed and engaged internal champions.
Sight Machine has made freely available a methodology for evaluating a manufacturer’s readiness for digital transformation. The methodology, called the Digital Readiness Index, is available at sightmachine.com/digital-readiness.
BUILD A PROPER DATA MODEL
Scaling digital manufacturing initiatives relies on a few critical areas of focus. From an IT perspective, handling the storage (Volume) and processing power (Velocity) needed for large amounts of information is typically front of mind. Of the three V’s of big data, the Variety of that information is typically a distant afterthought, if considered at all during the planning phase.
Forming a semantic model of a complex process such as a manufacturing line requires deep collaborative effort between those familiar with the technical tools for handling large amounts of data, as well as those well versed in the art and science of creating drug products. These are very different cross-functional skillsets, and many projects have failed due to the lack of alignment between Operations Technology and Information Technology.
Assuming OT/IT are aligned, enablement technologies can save appreciable cost and development time, as well as increase the likelihood of success in digital transformation. For instance, when looking at a number of manufacturing processes, it is challenging to ensure that a standard data model will apply to lines from dissimilar vendors, or those that produce dissimilar products. Interoperability of modeled data is critical for systems-level thinking. It is important not to fall into the project-based trap of doing one-off data projects, as this will curtail the upside from using digital technologies across the enterprise.
PHARMA/BIOTECH USE CASES
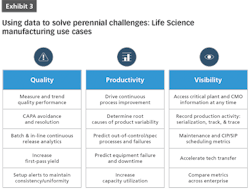
Data-driven decision-making is a powerful tool to address a wide variety of use cases in pharma and life sciences, including:
Cost Control
While there are still promising new R&D developments and the future of drug discovery is bright, there is a marked shift to cost control as the frequency of blockbuster drugs, and the associated margins, are decreasing.
Other industries, such as automotive, have been under significant cost pressure for some time. Lean thinking at each stage of the process is important for productivity gains, such as reduced scrap and increased throughput. Modern big-data techniques to model manufacturing facilities and apply statistical or machine learning methods can deliver profound increases to bottom line.
Technology Transfer
While a necessary step in scaleup, technology transfer can often be slow and cumbersome. A data-centric approach with real-time visibility can complement and accelerate such an effort. Instead of paying for employees to camp out at recipient facilities for extended time periods, companies can rely on data modeling to get real-time visibility into every remote parameter in order to complement an on-site team.
The real-time ability to track every process parameter in context, even if the source and recipient machinery are not identical, is a huge accelerant for tech transfer initiatives. An analytics approach allows for rapid correlation of machines within the process from the source and the receiving site. Dissimilar process parameters can be surfaced up for inspection, many of which are not available from the raw incoming data.
As an example, if there is an optimal time-pressure curve for every vial fill, a heuristic for the deviation from that optimal curve can be computed on a per-vial basis. Increased deviation at one site might correlate with increased scrap later in the process, perhaps due to bubbles affecting an automated visual inspection system. A modern data platform allows for rapid contextualization of the process, the ability to surface this correlation, and compare the process parameters between dissimilar sites, rapidly and in real-time.
Equipment Utilization
Data analytics can help manufacturers get more from their machines. Although pharmaceutical manufacturers have invested heavily in advanced machinery, equipment utilization in pharma is far lower than in other industries. An advanced visual inspection machine might analyze 600 vials a minute, but only runs infrequently throughout the day.
Across industries, an Overall Equipment Effectiveness (OEE) score of 85% is world class. In pharma, where traditionally costs have been centered in R&D and product development as opposed to manufacturing, it is not uncommon to have lines operate with OEE in the teens.
Guided by the data, there is large opportunity to improve productivity. Increasing the productivity of an existing line reduces unit costs of production, both from operation costs such as the people and energy required to run the machine for a given time period, as well as capital expenditures, as output from existing machinery is increased.
Supply Chain Complexity and Offshore Manufacturing
The need to reduce costs has increased the number of pharmaceuticals and active ingredients manufactured in developing countries is increasing. Maintaining effective quality control is compelling pharma makers to increase their level of visibility and monitoring of offshore manufacturing facilities. Additionally, real-time exchange of information from primary component and raw material suppliers can reduce incoming quality inspection costs and allow proactive decision making for routing upstream components to the most appropriate receiving site based on knowledge of batch-to-batch variation.
Precision Medicine
There is a shift away from one-size-fits-all to products aligned to the specific genetic makeup, health-related behavior, environment, and lifestyle factors for subpopulations or specific patients. This new tailored approach to medicine will require significant changes in manufacturing capabilities to enable custom products.
In other manufacturing verticals, the number of products produced can be large, with regular changeover. In high-mix verticals, it is not uncommon to see a million different products produced annually. In traditional pharma manufacturing, the number of SKUs is quite limited, and as a result, a line is set up to do the exact same thing for the life of the drug. With the introduction of medicine customized for individual patients, the complexity of the product mix and associated production process will increase. Dealing with the combinatorics challenges of customized medicine requires a data-focused approach.
Increased product mix will also have a profound impact on regulatory requirements, as many of the systems in place rely on rigidity in the process that will not be possible with highly variable outputs. Showing complete knowledge of the manufacturing process is required to ensure appropriate control in a varied environment.
Continuous Manufacturing
Continuous manufacturing processes will be reliant on analytical tools that are the next generation to Process Analytical Technologies (PAT) and Quality by Design (QbD). Correlation of process parameters with in-situ real-time measurements of critical product characteristics is now attainable, but rarely employed in practice in pharma manufacturing. For instance, downstream non-destructive tests such as weight or NIR particle measurements can provide closed-loop real-time feedback to the upstream blending stage to alter the process parameters in order achieve the optimal tablet consistency.
Regulatory Issues
The Drug Supply Chain Security Act requires that the industry implement end-to-end traceability by 2023. In addition to assuring compliance and continued product access for patients and customers, product-level serialization (unique tracking) can enable the investigation of counterfeit and diverted products, affording brand owners additional supply chain integrity and security. End-to-end visibility means that recalls, where necessary, can be executed more efficiently. All pharma companies are piloting efforts to improve product tracking and enhance visibility.
While simple traceability in an increasingly interconnected world is a good first step, is not the end state. Other verticals are becoming more proactive around data management. Auto manufacturers have had component level traceability for some time. More recently, they are not only tracing components throughout the supply chain, but are characterizing the production process parameters and relating them to each component at time of production. In the event of a quality issue or recall, the data has already been proactively organized for analysis instead of launching a retrospective effort to try and request and model the information in order to determine the root cause of a failure. Although not impossible in the past, this type of contextual modeling is far more approachable given recent technology advances and tools for working with information.
The analytical methods pioneered by consumer Internet companies like Google and Amazon are being applied across industries under the banner of digital transformation. Sectors including marketing and financial services have deeply embraced data-centric management practices. While organizational and technical challenges have slowed the embrace of big data in pharmaceutical and life sciences manufacturing, rapid technical advancements and marketplace pressures mean the digitization of life sciences manufacturing is now fully underway.