Embedding Regulatory Intelligence for Improved Change Control
Change control and variation management are areas where coordination between functional areas is critical to a company’s agility, profits and compliance. Yet information gaps often hinder collaboration. When a company wants to change an approved product, manufacturing process or supplier, it is a highly complex and lengthy process spanning multiple departments.
It’s a significant challenge in frequency and scale:
• Lots of changes. According to one expert, a top 20 company may evaluate more than 40,000 change requests in a single year, and approve approximately 15,000 changes that must be implemented across the organization. At some life sciences companies, a single product can have more than 200 changes a year.
• Lengthy cycle times. From initial assessment to final regulatory approval and implementation, changes can take several months, or even years, to complete.1
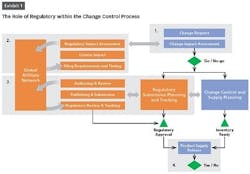
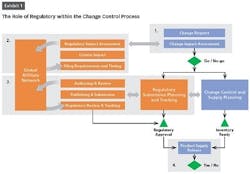
The change control review board (CCRB) is dependent on information from other teams such as regulatory, manufacturing and supply chain to make informed go/no go decisions on requested changes. Often, however, information from regulatory is significantly delayed because organizations lack the visibility and knowledge to reliably assess the impact of a change in other regions.
In this article, we’ll focus on how to improve the role of regulatory within the change control process so that the quality team or change control review board can make informed decisions quickly and speed submissions.
MAKING A GO/NO-GO DECISION
Assessing when and where to file a variation
As process owners evaluate the impact of a change request, they need to determine which regions would be affected and the types of filings required at each location. Countries have different variation requirements, and there is room for interpretation as to whether a filing is needed. Making the wrong judgment results in extra time, effort and cost.
Health authorities recognize the uncertainty and expense that companies face related to filing variations, and they are working on new recommendations for more clarity. The International Council for Harmonization of Technical Requirements for Pharmaceuticals in Human Use (ICH) expects to release the Technical Document for ICH Q12 for public comment in October 2017 as a step toward streamlining the way post-approval changes are managed.
A main element of the initiative will be to define “established conditions” for changes that require regulatory approval upfront, at the time of the initial licensing approval. Low risk changes will not be included as established conditions and will be documented - along with any other changes - in a company’s quality system.
ICH Q12 will also encourage manufacturers to define post-approval change management protocols (PACMPs) that describe how they will manage variations during a product’s lifecycle. While PACMPs exist in the U.S. and Europe, they have not been widely adopted. The ICH hopes to stimulate broader use - this is especially important when multiple specific changes are expected for a particular product.
Establishing consistency will speed change and instill greater confidence for regulatory bodies in the way companies handle variations. With ICH Q12, firms establish the regulatory intelligence up front to ensure they make informed decisions when filing variations.
Determining the scope of impact for variation filings
As companies consider a change, it’s challenging to assess the extent of its impact on the organization. A single ingredient or manufacturing change can affect multiple products and every license where they are marketed.
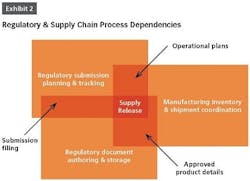
In many cases, regulatory depends on quality and supply chain information to determine which products are impacted by a change. With product registrations in the Regulatory Information Management (RIM) system, regulatory can quickly determine which products are affected by a change and where those products are licensed.
Regulatory teams must frequently contact affiliates for local licensing details. If affiliates were to maintain product licenses in an easy-to-use, shared global system, the team at headquarters could quickly run an impact assessment report across geographies. Also, headquarters could gather additional data as needed via trackable workflow tasks. This helps address one of the most common delays in the assessment process, by making it easier to gather information from affiliates and providing visibility into outstanding requests.
Evaluating the scale of a proposed change
Once regulatory knows which products and licenses are affected, they must estimate the amount of time and effort required to execute the proposed change. Compiling this information can require multiple phone calls, emails and searches through spreadsheets and mountains of paper. There is an opportunity to systematically, globally capture responses from regulatory professionals during impact assessments.
Some countries restrict sponsors to one active submission at a time. Tracking this information globally provides visibility into ongoing submissions, so expected delays can be incorporated into the broader decision-making process. It also provides a historical view into the length of time it took to make past filings in different markets.
With affiliates inputting information directly into the global system, headquarters can easily see if they have information for all countries or markets and estimate the resources and time it will take for a change to be implemented.
Making an informed cost/benefit decision
In addition to identifying criteria associated with a proposed change, regulatory should provide cost estimates. What may initially seem like an upfront savings to the company can end up costing more in the end, due to high levels of effort and expense incurred by the teams and regions filing the variation and implementing associated changes.
The more companies consolidate information and provide greater transparency, the greater insight individuals have throughout the process. During the assessment, teams can more easily evaluate data from the RIM system, and extrapolate that into cost calculations to help inform a go/no go decision.
SPEEDING THE VARIATION FILING PROCESS
Removing uncertainty during submission processes
When regulatory knowledge is integrated into processes and systems, global variation filings can be accomplished much more quickly.
“Companies would love to have better up-front information. Imagine if you could say ‘I want to submit to Bolivia,’ and real-time intelligence immediately showed here are the 10 documents that Bolivia will absolutely want for this type of change,’” said Bernie Coney, Head of Regulatory Advisory Services, at Kinapse, a life sciences consulting firm. “You could assemble the required materials much more efficiently.”
Dossier-level intelligence within a RIM system could embed that regulatory knowledge in submission templates. Companies have struggled with this challenge for years, relying on overly complicated Excel spreadsheets. Using pre-defined business rules to dynamically create a table of contents would significantly aid planning, coordination between regions, and the review process for a submission and its components.
There are many different types of ancillary documents, and requirements vary by country. While this content is relatively standardized for major markets, headquarters may not know what is required by other countries until the local submission is initiated. This can immediately put the process behind schedule. Ideally, submission content plans generated by the RIM system would surface ancillary documents required for submissions around the world, streamlining what is often a complicated and reactive process.
Applying supply chain thinking
The auto industry uses a supply chain approach to orchestrate on-time delivery of required components. Harry Smyser, an independent consultant at Kinapse with 25 years of experience in supply chain and regulatory functions, believes that life sciences companies can apply similar methodologies to assembling variation filings.
Regulatory can manage ancillary documents holistically, with dependencies and idiosyncrasies factored into the process from the onset. “If I’m making an equipment change for a product that is manufactured in France but sold in Mexico, the Mexican agency will need a certified pharmaceutical product (CPP) from France attesting to the fact that the manufacturing site has passed inspection,” said Harry Smyser. “Getting a CPP from France can take six months. That process should be initiated as soon as the change is green-lighted by the review board, instead of waiting until the core submission documents are ready.”
Ensuring that documents are current and accurate
When assembling submissions, it is difficult for regulatory to know whether the content within the documents is still accurate. Text, graphs and charts are manually copied and pasted so many times that there’s no easy way to verify that the data is current. And since affiliates may be assembling their submission a month or more after the original filing, they must re-confirm whether updates have been made in the interim.
In many Latin American countries, the bundling and unbundling of change requests makes it even harder to know what’s current. Not only do bundled variations introduce delay, the bundled submission differs from the individual component submissions. The delays caused while affiliates validate information or update outdated charts can have a dramatic impact on timing and revenue.
When document information is turned into structured content or data, it can be managed with more precision. Having an authoritative source with granular control ensures accurate and up-to-date information is maintained across multiple submissions, significantly reducing cycle times and eliminating the need to re-run reports or search for updates.
MANAGING INVENTORY AND PRODUCT SUPPLY RELEASE
Product release is an activity at the end of the change control process where poor visibility to regulatory information can have big repercussions. Companies must balance the timing of product releases, production change-overs and inventory levels across impacted markets. Lack of coordination between regulatory, quality, manufacturing or supply chain teams can lead to higher production costs, product shortages and compliance issues.
Optimizing inventory management
Companies can only release updated product to a market after it has been approved by the relevant health authority. Firms work hard to prevent mistakes and avoid sending updated product before it is approved, but it gets complicated by the vast number of variations submitted each year.
The length of time it takes to secure regulatory approvals across each market can have an impact all the way down to the production line. Accurate demand forecasting is essential to optimize production and minimize time spent running dual production lines while waiting for approvals to take place.
Improving the timing of supply release
To effectively manage supply release, approvals must be tracked globally and reflected in product registration details. That information needs to be readily available to quality teams or “Qualified Persons” (QP) in Europe.
This requires affiliates to track the status of variation filings in a shared system so that the global view into approvals stays current. Grouping a variation submission and its associated communications by health authority helps regulatory keep track of what has actually been approved.
Companies also need a systematic approach for sharing approved data with quality teams. Processes that are dependent on emails and phone calls are impossible to scale and introduce risk for human error. Sharing registration details via reports or direct access can save a great deal of time and back and forth between groups.
For example, to make information transparent to the right audiences, UCB, a multinational biopharmaceutical company, began issuing automated reports showing the current state of approvals. This replaced ad hoc email exchanges with a reliable, weekly flow of information. QPs releasing product to market now have current and accurate data, ensuring that the product they are releasing matches exactly to the information on file.
Moving forward, UCB would like to deploy a dashboard or other real-time approach. One method is to grant quality individuals or the QP direct access to the RIM system, where the individual can view dashboards with the status of regulatory approvals or reports with relevant product registration details. Security controls can be configured so that only appropriate data is shared, preventing any misunderstandings related to confidential information.
Another method is to grant quality individuals or the QP direct access to the RIM system, where the individual can view dashboards with the status of regulatory approvals or reports with relevant product registration details. Security controls can be configured so that only appropriate data is shared, preventing any misunderstandings related to confidential information.
The best strategy is standardizing regulatory and quality applications on a common platform, allowing relevent regulatory information to be accessed by the quality system to seamlessly support decision-making. This approach simplifies processes and eliminates the challenges with maintaining integrations and a validated environment during system upgrades.
Quality teams and QPs depend on information from departments beyond regulatory to fully implement a change. Moving toward a harmonized and consolidated dashboard that pulls data from related departmental systems - ERP, labeling and others - is a more effective strategy for keeping quality and the CCRB updated.
By bringing regulatory and quality teams closer together with regulatory information accessible throughout the change control process, companies can transform change management from a burden into a competitive advantage.
Companies can dramatically improve efficiencies and speed variation filings with integrated information and processes. Whether submitting variation proposals or making product ship decisions, teams can trust that the data is correct. For a large organization, these benefits translate into millions of dollars each year, and more than justify the effort in leveraging regulatory intelligence to improve the change control process.
REFERENCES
The Future of Post-Approval Change Management. Sarah DeMare. December 2016. Retrieved from http://virtualregulatorysolutions.com/the-future-of-post-approval-change-management.