There are strict regulations on the permitted levels of elemental and chemical impurities within manufactured drugs. The presence of an impurity can lead to significant side effects. This means the analysis and monitoring of impurities is paramount during drug development and the production process. Here we explore the use of X-ray analysis in detecting elemental impurities, and highlight the benefits of X-ray methods in comparison to other techniques.
X-RAY POWDER DIFFRACTION
The use of X-ray analysis and its benefits within certain parts of the pharmaceutical industry are well established. X-ray powder diffraction (XRPD) has become a recognized and reliable technique for quantitative and qualitative analysis of crystalline drugs within the industry, including testing for unwanted compounds. Indeed, XRPD data forms a part of all regulatory submissions on a new compound.
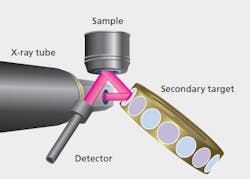
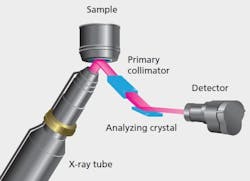
Figure 1: Schematics illustrating EDXRF optical geometries.
X-ray powder diffraction has been the gold standard technique for qualitative and quantitative analysis of crystalline compounds for many years, ever since it was admitted to the United States Pharmacopeia (USP) in USP 25-NF 201. The main application for XRPD in the pharmaceutical industry is to study polymorphism. Most drug molecules are formulated in a crystalline form and have the ability to crystallize into a wide range of structures known as polymorphs. Different polymorphs have different chemical and physical properties; therefore, it is essential that the polymorph present in the final drug product is controlled. The key benefits to XRPD when compared to other techniques for studying crystalline compounds include simple sample preparation and non-destructive analysis. The technique and the technology has matured, with ongoing development leading to lower detection limits, faster measurement times and a wider range of possible applications2.X-RAY FLUORESCENCE SPECTROMETRY
The closely related technique X-ray fluorescence spectrometry (XRF) has recently been incorporated into the USP as chapter <735> X-ray fluorescence spectrometry3. XRF is well established in a number of industries as a technique to determine elemental composition, including quantifying elemental impurities. XRF provides numerous advantages in production and quality control, including less need for re-calibration and low total cost of analysis, as well as making it possible to automate analysis. XRF is a valid replacement for the current wet chemical techniques which, until recently, were one of the few USP approved methods for impurity analysis.
XRF is a non-destructive analytical technique used to identify and determine the concentrations of elements present in solid, powdered and liquid samples4. XRF is capable of measuring elements from beryllium (Be) to uranium (U) and beyond, at trace levels often below one part per million and up to 100%.
XRF spectrometers measure the individual component wavelengths of the fluorescent emission produced by a sample when irradiated with X-rays. The X-ray fluorescence technique is both a qualitative and quantitative method. The presence of peaks at specific energies is indicative of the presence of the elements in the sample, while there is also a direct correlation between the intensity of the peaks and the concentration of the elements in the sample.
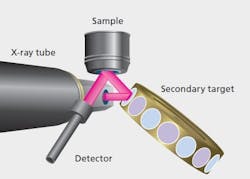
XRF instrumentation is conventionally divided into Wavelength Dispersive (WDXRF) and Energy Dispersive (EDXRF) instruments, the distinguishing factor being the technologies used for the energy discrimination and detection of the X-ray photons. Figures 1 and 2 show schematically the optical geometries for WDXRF and EDXRF. WDXRF instrumentation typically has much higher power loading on the sample than EDXRF. This may limit the use of the WDXRF technique (typically with power in kW range) for organic matrix sample analysis because the heat generated by the X-rays may be sufficient to induce sample alteration, such as browning or discoloring of the sample surface or loss of volatile elements, for example, Hg and Se. EDXRF systems, on the other hand, use low power X-ray sources or secondary targets and will not significantly heat the sample. As the bulk of pharmaceutical materials are organic in nature, the focus of this article is to emphasize the usefulness of the EDXRF technique, which has the best potential for the measurement of all elements from Na to U in pharmaceutical samples.
ELEMENTAL IMPURITIES
Recent developments in the pharmaceutical industry are changing the way pharmaceutical companies approach testing of elemental impurity analysis in drug substances, excipients and drug products. USP chapter <231> heavy metals is going to be removed from the pharmacopeia and it will be replaced with two new chapters: <232> Elemental impurities — limits, and <233> Elemental impurities — procedures5. Additionally, the USP has been working together with the European pharmacopeia and the Japanese pharmacopeia as part of the International Conference for Harmonization to produce the new ICH Q3D guideline for elemental impurities.
Figure 2: Schematics illustrating WDXRF optical geometries.
The new USP chapters and ICH guideline introduce the idea of a risk-based strategy to outline the elements that need to be controlled, list the permissible levels for these elements in final drug products, and suggest suitable analytical techniques for testing materials. The ICH Q3D guideline states that “Pharmacopeial procedures or suitable alternative procedures for determining levels of elemental impurities should be used”⁶. USP<233> details two compendial procedures for determining elemental impurity content, while also stipulating that alternative procedures may be used providing they are validated and meet the acceptance criteria⁷. The availability of alternative procedures allows companies to decide upon their own methods of testing to suit their needs, making way for new technologies like XRF to be utilized, explored and further developed.The two compendial procedures detailed in USP<233> are based on the principle of inductively coupled plasma (ICP), which typically uses a metal coil conducting a large current with flowing argon gas to produce extremely hot plasma⁸. The hot plasma generated is then used to excite and ionize the elements in the sample, which can then be detected using atomic emission spectroscopy or mass spectrometry.
ICP-based techniques are extremely sensitive with very low detection limits - in the order of parts per billion - significantly lower than what is required by the new regulations. However, there are a large number of drawbacks which make them less than ideal for pharmaceutical elemental impurity analysis. One of the largest drawbacks of ICP techniques are the sample preparation requirements. ICP requires all samples to be introduced as a liquid, which for most pharmaceutical materials requires a dissolution or digestion step. Additionally, the sample usually requires further dilution steps to ensure that the concentration is within the working range of the ICP instrument. This complex sample preparation can take many hours and lead to unnecessary errors. Another large drawback is related to total cost of ownership and operation. As it is a very sensitive technique, ICP instruments require special installation considerations and highly trained operators; there are also ongoing costs for argon and consumables.
X-RAY’s FUTURE
X-ray analytical technologies play an important role in pharmaceutical development and this role is going to expand with the recent addition of X-ray fluorescence to the United States Pharmacopeia. X-ray techniques provide fast, non-destructive analysis with reduced chemical waste and, compared to some techniques, lower total ownership costs. The new addition of XRF provides pharmaceutical companies with another method to consider for elemental analysis, including compliance to the new elemental impurity USP and ICH regulations.
REFERENCES
1. United States Pharmacopeial convention (USP 25-NF 20), chapter <941>, 2002.
2. An Interview with: Dr. Julie Quinn, American Pharmaceutical Review, October 2015, p106 – 107.
3. United States Pharmacopeial convention (USP 37-NF 32), chapter <735>, 2014.
4. Brower P., Theory of XRF: Getting acquainted with the principles, 3rd edition, 2010.
5. United States Pharmacopeial convention, Revision Bulletin, March 2015
6. ICH Q3D Quality guidelines, (www.ich.org/products/guidelines/quality/article/quality-guidelines.html).
7. United States Pharmacopeial convention (USP 35-NF 30), chapter <233>, 2012.
8. United States Pharmacopeial convention (USP 37-NF 32), chapter <730>, 2014.