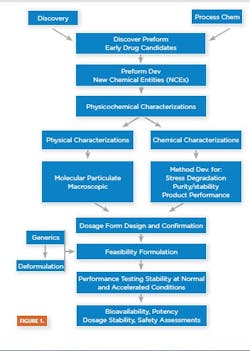
Drug product predevelopment activities deal with the creation of baseline data necessary for the formulation of safe, effective, efficacious and stable drug products. Adequate and timely predevelopment activities ensure a drug product that meets safety, stability, quality, identity, purity and potency (SSQUIPP) requirements and is fit for its intended purpose.
Predevelopment tasks involve all the activities aimed at establishing API/Excipient physicochemical properties: pre-formulation and feasibility formulation to aid in the selection of desirable dosage form [6.1] ; feasibility process development and scale-up to aid in the selection of desirable processing equipment and train; a sterilization study aimed at determining the most suitable means of achieving drug product sterility (for sterile products) [6.2] and a feasibility of primary packaging study [6.3], aiding in the selection of primary and secondary packaging equipment and train. Predevelopment activities also support determining overall process configurations with quantified critical process and quality parameters for eventual clinical and commercial manufacturing processes.
Dosage Form Design and Confirmation
Prior to starting feasibility formulation development, the baseline information/data generated on the new chemical entities (NCEs) — particularly the chemical structure, spectral data melt and transition properties — are compared to a library of compounds for any closely related compound(s). This, coupled with past development experience, helps in designing physicochemical experiments to determine the type (acid, salt, crystal, polymorph amorphic) and the most appropriate dosage form for drug product development. The dosage form stability and performance are confirmed as the product development is progressed through the various stages up to commercialization.
Feasibility Formulation and Feasibility Process Development
Formulation is the process in which different chemical substances, including the active drug, are combined to produce a final medicinal product [6.5]. Feasibility formulation studies involve developing small or bench-top formulations for physicochemical and drug-excipient compatibility screening to aid the dosage form stability evaluation, phase I clinical and eventual patient acceptability assessments.
After bench-top feasibility is confirmed, feasibility process development can be initiated. Process Development is a series of predefined experiments to identify critical variables in a process and determine how these variables may influence scale-up [6.6]. It is an essential step leading up to formal process validation and post-validation activities. Feasibility Process Development defines and tests desired operating parameters, which include the formulation composition, addition sequence, mixing speed, time, evaluation and the design of processing equipment.
Feasibility Sterilization Process Development for Sterile Products
Sterilization can be achieved by applying the proper combinations of heat, chemicals, irradiation, pressure and filtration. Feasibility sterilization studies are aimed at development and assessment of the sterilization method that achieves the primary objective of eliminating all microbial life from a product without effecting dosage form and formulation components or overall product quality.
Terminal sterilization is the process in which the finished pharmaceutical product is sterilized in-situ in the final container meant for delivery to the end user [6.7]. Two basic approaches are employed: Overkill and Probability of Survival. The Overkill method is used when the product can withstand excessive exposure to the sterilization processes without adverse effects. The Probability of Survival approach is used when there are limitations to exposure to the sterilization parameters that the product can withstand. In the probability approach, the process for the terminal sterilization is validated to achieve the destruction of the pre-sterilization bioburden with a minimum safety factor of an additional six-log reduction (1x10-6). The probability that no more than one unit per million is contaminated is considered to be an acceptable level of sterility assurance.
Due to API/excipent sensitivity to heat, some products cannot be terminally sterilized and must be aseptically prepared [6.8]. For heat-sensitive formulations, the product may be sterile filtered, however, sterile filtration cannot be applied to certain formulations, including suspensions or emulsions because sterilizing grade filter membranes with sub-micron pore sizes that remove microbial contaminants may also screen out API particles or micelles.
When sterile filtration is appropriate for liquid formulations [6.9], the affinity of the formulation components to the filter membrane, as well the degree of adsorption and/or absorption of the components to the membrane, become critical to the process and quality of the sterile product. For bulk sterilized products, the sensitivity or stability of the API/product to the applied heat or radiation is again critical to the overall process and product quality.
Feasibility Filling and Packaging Process Development
Primary and secondary packaging process development planning depends on the dosage form, appropriate filling and packaging equipment required, container and closure system and overall finished product presentation desired. The nature, complexity, costs and duration will differ among the various dosage forms. It is critical that the container and closure system be developed to fit the dosage form, delivery routes and planned presentation. It is equally important that the filling and packaging process equipment train be developed to ensure not only compatibility with the product, container and closure system, but also consistency in fill weight or volume to yield the desired delivered dose of the product. Container integrity needs to be assured as well. With the proper application of filling and packaging equipment suitable for the desired container/closure system and by controlling the filling and sealing of the containers and closure systems, the reproducible production of a product can be assured.
Predevelopment activities impact to SSQUIPP
Safety — The physicochemical properties of a drug substance influence its subsequent development and dosage form selection. Even when a drug substance passes early toxicological tests, there remain considerable safety issues to be considered and addressed all the way to the market.
Drug toxicity may be influenced by many factors. Sound scientific predevelopment and feasibility development activities can help reduce or eliminate these potential toxicological hazards and enhance product safety through extensive characterization and the correlation of the drug product in vitro data to in vivo performance.
Stability — Drug stability can be impacted by the processing conditions used to produce the drug. For example, a drug substance can exist in two or more crystalline phases that have different arrangements and/or conformations (forms) of the molecules in the crystal lattice. Depending on the processing conditions of temperature, solvent selection, processing time and formulation controls, these forms can change from one crystal form to the other or can become amorphous. The process is called polymorphic transition. Critical polymorphic transitions may be accompanied by change in particle size or melt and transition properties such as segregation, precipitation or sedimentation. These changes can affect drug substance activity as well as result in adverse impact on drug product stability.
Polymorphs have different stabilities and may spontaneously convert from an unstable form to the stable form at a particular temperature. They also exhibit different melting points and solubilities, which affect the dissolution rate of drug and consequently bioavailability in the body.
Many compounds absorb water vapor or moisture. This absorption (or hygroscopicity) affects API/product stability. Understanding hygroscopicity during the formulation and process development phase is key in determining process and product controls including handling, storage, and critical processing conditions. APIs can be classified into slightly hygroscopic, very hygroscopic and deliquescent.
Predevelopment activities, including identification and control of critical process parameters are designed to determine the most stable form of a polymorphic drug.
Quality — Pharmaceutical quality has many definitions, but pharmaceutical quality as specifically applied to drug product quality includes assurances designed to produce a drug product that is free of contamination and reproducibly delivers the therapeutic benefit promised on the consumer label. [6.10] All properties that impact drug product SSQUIPP also impact on drug product quality.
Process-related physical properties can influence particle size, handling methods, manufacturability, API specification, labeling, packaging and storage condition requirements, as well as other key product criteria.
Early characterization and control of drug chemical properties relating to degradation, reactivity and compatibility under normal and stressed conditions favorably impact drug stability. Sound preformulation, feasibility formulation and process development involve activities that help ensure the development of a drug product that meets safety, stability, quality, identity, purity and potency (SSQUIPP) requirements through the application of quality by design [6.11].
Identity — Typically, drug identity refers to labeling considerations. But Identity issues can arise when the drug product is not what it is claimed to be. Identity issues can be avoided by following correct standard operating procedure for manufacturing, storage, distribution and administration of approved drug products as well as ensuring strict compliance with all GMP and regulatory requirements.
Purity — Impurities are foreign materials not part of the defined drug substance, excipients or other additives. There are many sources of impurities, including those from the raw materials (drug substance, excipients or other additives), residual solvents, degradation products and foreign materials.
Impurities can also arise from exposure to light, heat, humidity, container or label interactions, pH drift during storage and other means. Impurities can also arise from crystallization or recrystallization of drug substance during processing or storage, residual solvents or other by-products of the API manufacturing process. Predevelopment experiments are designed to assess the potential sources of drug impurities and to determine remediation steps to avoid impurity prone processes or interactions in the future.
Potency — There are several physical factors in the production setting that may impact drug potency including weighing mistakes, and losses during processing (transfer losses, sorption losses (adsorption or absorption). Process equipment trains can produces losses as well. Other factors, such as polymorphic transitions may reduce potency. Degradation and /or drug-excipient interactions may impact potency claims as well. Here, sound predevelopment and feasibility development procedures can help detect potential product potency variables, identifying remedial measures and support consistent production of drug product at the targeted potency.
Development Costs and Time to Market
The main objective of conducting predevelopment activities is to produce and deliver a drug product that meets SSQUIPP requirements to the end user. Failure to perform well-planned predevelopment activities early in the development stage is likely to lead to time-consuming and costly delays [6.13]. There are enormous cost implications involved with poorly executed drug product development. Mistakes or missteps resulting from improperly planned and executed physicochemical characterization or process development studies can raise development costs dramatically. Similarly, incomplete product knowledge stemming from poorly executed development studies instead of systematic and deliberate creation of product quality suitable for intended patients by design has the real potential to delay or derail a drug product launch.
Product development delays and costs most likely will increase if there is not a well-planned and systematic approach to development challenges. These challenges can be addressed by engaging in a systematic and deliberate process to ensure product quality suitable for intended patients by design. This QBD approach requires a thorough understanding of the physical properties of drug substance and excipients — knowledge obtained by exhaustive characterizations of API, excipient and feasibility product during predevelopment stage. The baseline data generated at this stage is critical for the formulation of a stable, efficacious and safe dosage form compatible with delivery system.
The advantages of well-planned and executed predevelopment activities are therefore not only realized in minimizing the potential for costly delays to drug development but also has direct impact on product overall quality (SSQUIP), reduction of product development costs, acceleration of time to market and getting planned activities right the first time.
References
6.1 G. Steele, “Preformulation as an Aid to Product Design in Early Drug Development,” in Pharmaceutical Preformulation and Formulation: A Practical Guide from Candidate Drug Selection to Commercial Dosage Form, M. Gibson, Ed. (Interpharm Press, Denver, CO, 2001), pp. 196–210.
6.2 J. Sharp, “What Do We Mean by ‘Sterility?” PDA J. Pharm. Sci. Technol. 49 (2), 90–92 (1995).
6.3 C.P. Croce, A. Fischer, and R.H. Thomas, “Packaging Material Science,” in The Theory and Practice of Industrial Pharmacy, L. Lachman, H.A. Lieberman, and J.L. Kanig, Eds. (Varghese Publishing House, Bombay, India, 3rd ed., 1991), pp. 711–732
6.4 G. Brittain (Ed.), P1-34 “Polymorphism in Pharmaceutical Solids”, Marcel Dekker, Inc., New York, 1999
6.5 W. Lund, (Ed). Principles and Practice of Pharmaceutics, The Pharmaceutical Press, London, UK, 12th ed., 1994
6.6 PDA Journal of Pharmaceutical Science and Technology, Technical Report #42, Process Validation of Protein Manufacturing, Supplement Volume 59, pages 3-7, September/October 2005.
Published in the February 2013 issue of Pharmaceutical Manufacturing magazine