Using Quality by Design to Optimize Tech Transfer of a BFS Process
Technology transfer is a difficult and sensitive process. For a drug service supply partner, transferring an existing drug product from a customer’s facility to its own manufacturing plants offers an opportunity to increase value by improving process efficiencies and equipment design, and to incorporate operational excellence principles into the work.
Using the principles of pharmaceutical Quality by Design (QbD) can help set guidelines for tech transfer, and ensure that opportunities for process improvement are not overlooked when focusing on budget and deadline.
This article will summarize how Catalent worked within the QbD framework to help a customer transfer a new blow-fill-seal (BFS) process.
In 2010, Catalent was approached by a Fortune 500 drug manufacturer to transfer an existing ophthalmic product from a manufacturing site Catalent’s BFS facility in Woodstock, Illinois. The product was already marketed commercially and the FDA approval for Catalent manufactured product was to be based on the manufacturing process used at the customer’s site.
Once the site was selected, the technical transfer process began with a detailed comparison of the client’s current manufacturing process vs. the process that Catalent had initially proposed.. A transfer document was created that retained the critical process parameters, while identifying areas of improvement. In this project, opportunities were identified in the following processes:
• Optimize the formulation from a three tank process to a two tank process
• Incorporate an increased level of automation to the formulation process
• Redesign of the unit dose BFS ampoule to minimize the probability of leakage
• Redesign of the label and the label application process
• Redesign of the foil pouch to reduce scrap experienced at the original site
Per ICH Q8(R), Quality by Design is a systematic approach that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management.
Using this definition, the first step of the technical transfer is to create an outline or process flow of the existing process with critical parameters and expected results.
The outline should also include examination of the ingredients (Active Pharmaceutical Ingredients (API) and excipients) in the formulation to understand any special handling requirements or attributes associated with the materials.
This product contained two APIs and a polymer solution composed of two grades of the same material. In the course of the discussion, the customer mentioned a concern over the incorporation or mixing of the polymer solution. In their experience with this material, the polymer formulation was prone to the formation of globules that could cause inconsistent viscosity results, a critical process parameter, and potential clogging of the clarification filters used in the filling process.
In addition, in the product manufacturing process, a polymer solution must be bulk sterilized prior to addition of the API solution using sterile filtration. The high viscosity of the polymer and the uncertainty of the filtration process led the original site to develop a three tank process: a formulation tank for the polymer base with bulk sterilization capability, an API formulation tank, and a third tank to pre-filter the polymer into prior to combining with the API solution.
Both customer and drug service supply partner performed a risk assessment on the formulation process using a standard approach of assigning values to each step of the process for the rate/likelihood of occurrence, the ability to detect the problem, and the severity of impact to the process in order to properly design a formulation system based on the severity and probability of occurrence.
Catalent engineers believed that the process should dictate the design of equipment, and that a sound process combined with the proper equipment design would be robust enough to overcome any operator variability introduced by personnel performing a task. For this product, the highest risk to the process was the formulation of the polymer solution.
A solution for the inconsistency in the incorporation of the polymer was presented in the design of a highly automated two-tank formulation system. The polymer formulation involves the mixing of two lightweight hygroscopic powder ingredients into Water for Injection (WFI) at a high temperature.
The mixing vessel must have the proper agitation system that creates the proper vortex to incorporate the powder, or the powder will float on the surface of the WFI and form globules. The Catalent team designed a polymer tank that uses a standard top down mixer and also features a side scrapping system to aid in the incorporation of the powders. The side scrappers also prevent scorching of the polymer base against the heat transfer surface during the bulk sterilization process.
In addition, the two-tank process lowered the investment cost for capital equipment by the customer and significantly reduced the necessary floor space in the formulation area. The design reduced the amount of automation needed, and eliminated the loss of polymer solution inherent in transferring material between tanks.
By automating the formulation process, any variability introduced by the operators was greatly reduced. Critical parameters such as temperatures, mixing speeds, and mixing times are PLC controlled and monitored, rather than being dependent on operator manipulation. The sequencing of valves throughout the process is automated and programmed to prevent inadvertent opening or closing of pathways vital to maintaining sterility.
During the site acceptance testing and engineering tests of the formulation skid, simple Design of Experiments (DOE) were utilized to determine the optimal mixing speeds and durations for each of the polymers.
The tests were performed using multiple operators to add the powders using techniques varying from a slow addition using a scoop, to rapid addition by pouring the entire volume of powder into the vessel as fast as possible.
Various shaft lengths and impeller designs were tested prior to selecting a combination that would work with the range of operator induced variability possibly introduced during mixing. These mixing parameters were then programmed into the automation controls of the skid and incorporated into the Master Batch Record (MBR) for the product.
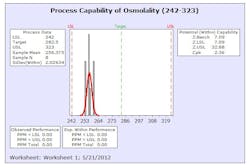
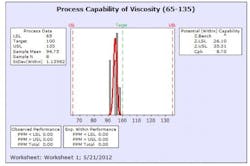
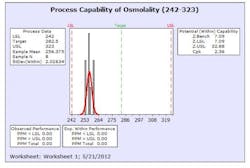
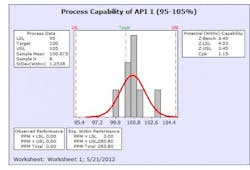
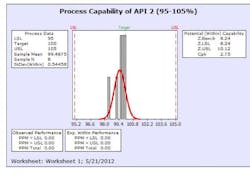
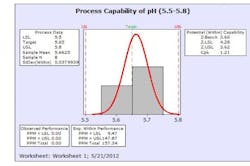
The results from this design process can be seen in the following graphs from the first eight production batches using this formulation system. The graphs display the viscosity, pH, osmolality and the assay values for the two APIs in the formulation. The batches were produced using regular production operators trained on the equipment and the MBR.