Optimizing Coating Parameters to Improve Tablet Performance by Means of Terahertz Pulsed Imaging (TPI)
Oral drug delivery is the largest proportion of the drug delivery market. The level of ease-of-use and cost benefit of the oral drug delivery market is beneficial to pharmaceutical companies in order to extend the revenue-earning lifetime of their major products. Traditional and non-complex oral drug formulations, though, cannot be used widely to provide the requested control over drug delivery. Controlled release and combination therapies are then used to provide the currently needed level of precision of performance for regulating the release of the active ingredients into the body.
Furthermore, great benefits are found in functional coatings when companies need to re-formulate a product; more and more drug makers are turning to reformulation to prolong the lifecycle of their top sellers and protect precious revenue from generic copies. As an example of this, between 2002 and 2005, 39 per cent of the total products from the top 50 manufacturers launched by pharmaceutical products were reformulations 1.
This is not surprising when thinking that reformulation of a product is the most effective way to prolong a product`s commercial life and it provides market share protection where generic drugs have become a natural feature of the pharmaceutical scene with branded drugs coming off patent each year and market exclusivity time decreasing.
In addition, pharmaceutical companies protect their products from generics by building complexity into the development and manufacturing of a product to make sure that it is strongly patent- protected when expiry of the initial product patent occurs. Functional coatings (as sustained release or oxidation protection coatings) being technologically advanced and complex products, are widely used to obtain this patent-protection strategy.
Finally the importance of functional coatings is also found when there is the need to protect the stomach to being exposed to high concentrations of active ingredients, or to improve tablet visual appeal and, finally, extend shelf life by protecting the ingredients from degradation by moisture and oxygen.
All the above benefits of functional complex coatings come at a price. The added complexity causes difficulty in scale-up and manufacturing. Combining this factor with having to move the products to market quickly we are left with a common scenario of batch failures during scale-up and, occasionally, the inability to manufacture consecutive batches reproducibly. In order to prevent and avoid any scale-up, manufacturing issues, precise and reproducible ways of measuring coating quality are needed.
Until recently the ability to non-destructively and quickly measure these coating and core critical parameters was impossible. Generally, to verify that the coating is applied in the correct amount to a tablet the average weight change post-coating is measured. Following this methodology we are only relying on the assumption that the coating is applied uniformly and that it coalesces in the same way each time. Weight gain can only give some guidance on what has happened with the coating process but it does not give an idea as to what has happened during the coating process and it does not give any idea of parameters such as uniformity of the coating and other parameters that can have a critical effect on the rate of the drug release such as density of the coating. As an example, in a coating process a sample of tablets can show and average weight gain of 6 per cent where in reality this can range anywhere between 4 and 8 per cent. If the dissolution and rate of drug release is reliant on this coating thickness, then the variance in dissolution will be very wide.
Some analytical and imaging techniques have been used to understand the critical processes involved in tablet coating, but none are ideal to fully characterise the layers. NIR and Raman are restricted to the outer surface of the tablet. To test the coating for uniformity or to analyse buried structures the tablet must be cut open. A non destructive alternative for characterising only density variations within a tablet, is X-ray microtomography. The downside of this technique is that it measures only physical properties and it requires long acquisition times, it is computationally challenging and radiation-induced strain in the sample is a possible consequence in each measurement.
For the above reasons, terahertz pulsed imaging (TPI) has become increasingly popular in order to monitor the key quality attributes in coated tablets. It can quickly and non-destructively probe the structural features of simple or more complex, multilayer tablets. Additionally TPI is an absolute method that uses low power, non-ionizing radiation at lower frequencies than traditional infrared techniques not affecting the sample in any way. Furthermore, as the macroscopic structure within the tablet coating and core is less than the wavelength of the radiation, scattering is not significant.
Terahertz Pulsed Imaging
Terahertz pulses incident on a tablet surface penetrate through the different coating layers. At each interface or change in refractive index, a portion of the terahertz radiation is recorded as a function of time. The technique operates much the same way as ultrasound or radar is used to accurate locate embedded or distant objects. In other words, a terahertz beam is scanned across the tablet which reflects back portions of the beam from the surface as well as from internal points in the dosage form where there is a change in refractive index. The delay of the returned pulse gives depth information.
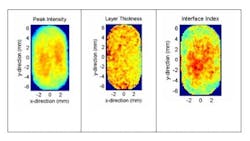
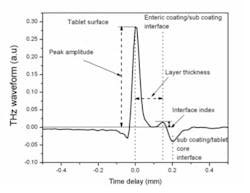
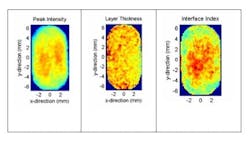
The sample itself is unaffected by the measurement and critical quality attributes can be directly measured without modifying or destroying the tablet. Figure 1 shows a typical waveform obtained from one single pixel when the terahertz beam is recorded. The various key metrics that are recorded from each waveform (peak intensity, coating thickness and interface index, as examples) can then be mapped in 2D and 3D images of the tablet.
Examples of these maps are shown in figure 2 with the definitions of each parameter.
Coating thickness uniformity is established by the transit time of the pulse to each interface. It is the high sensitivity to refractive index change that enables terahertz pulsed imaging to be used to map coating thickness.
It is important to emphasize that any number of locations can be probed by TPI within the tablet, and the measurement can be extended to produce a three-dimensional map of the tablet, showing tablet uniformity as a function of depth below the surface.
These precision measurements of tablet coatings are essential to pharmaceutical companies in order to accelerate drug development and improve process control and production consistency where tablet coatings are used as a key route to regulating the release of active pharmaceutical ingredients (APIs) in controlled or sustained release formats.
Non-uniformity in the coating can lead to serious consequences affecting safety and efficacy, making it vital for pharmaceutical companies to be able to assess coating thickness and uniformity as a quality control measure, both by batch and on an individual tablet basis. For this reason, terahertz can be seen as an incredibly strong tool to operate at the level of FDAs initiatives such as quality by design (QbD), seeking to ensure that the design and function of new solid dosage forms are optimised to de-risk process scale-up and eliminate conventional root cause analysis.
As the majority of pharmaceutical tablet coating and core material are semitransparent to terahertz the beam can penetrate depths not achieved by other methods of analysis and it achieves impressive thickness resolution of less than 40 m with accuracy of less than a few microns. For this reason TPI enables pharmaceutical companies to accurately determine coating thickness and uniformity; asses quickly and non-destructively structural features such as core integrity and API or excipient agglomeration and it is widely used to identify cracks, dislocations and delimitations as deep as 2 to 3 mm into the tablet core.
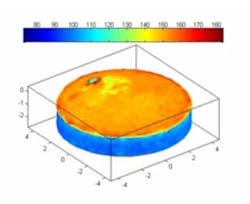
Figure 3 shows a typical 3D image of the tablet coating thickness. Nevertheless it is fast enough to be applicable to in-line measurements.
Terahertz radiation thus has enormous potential as a tool yielding new design information and quality assurance capabilities. Recent FDA studies have highlighted the correlation between coating and variance in dissolution in mesalanine tablets2. Mesalanine is a delayed release product enteric coated with a pH dependent acrylic-based polymer film. The coating is resistant to low pH so that it can pass through the stomach but then it starts to dissolve at pH 7 and above.
The product was demonstrating a high level of variability in dissolution. The FDA chose to investigate this behaviour with regard to the coating thickness.
From the study using terahertz imaging they found that when tablets failed the desired dissolution profile this could be linked directly to the variability in the coating thickness around the wall of the tablet.
The study went on further to demonstrate that the dissolution rates could be correlated to the average thickness of the tablet across a number of batches.
The study demonstrated that the coating thickness and uniformity could give an indication of dissolution behaviour, but that it also gave a much more accurate measurement of quality for the finished product than the usual measurement of weight gain. In the FDA paper it was stated that the speed and ease of the terahertz imaging “may make it an attractive replacement for wet dissolution testing both in product development and eventually for process analysis”.
In other studies on different enteric coated tablets 3 it was shown that TPI could reveal the changes in the interface between enteric and subcoating as the process conditions change (i.e. coating viscosity and spray rate). This difference in the interface indicated a change in adhesion between the two coating layers.
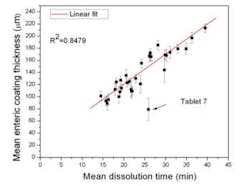
Further recent studies 4,5,6 also have further proved that a number of key parameters obtained by TPI can be correlated to dissolution. An example graph of correlation between coating thickness and mean dissolution times is shown in Figure 4 where non-destructive results of coating thickness by TPI, determined for each individual tablet are plotted against mean dissolution time. The tablet identified as tablet 7 is an obvious outlier. Tablet 7 shows the thinnest subcoating.
Conclusion
Terahertz pulsed imaging is currently being used extensively to investigate solid dosage forms and it can soundly assist in improving process understanding and define the correct manufacturing for a particular dosage form. It can also be used as a tool in the development, scale up and quality assurance of dosage forms, resulting in faster times to market and a reduced risk of regulatory non-compliance and product being kept off the market.
TPI is a unique tool to non-destructively measure the coating thickness that can be correlated to both the mean dissolution time but also to onset of dissolution important for enteric coated products and it has the potential to be used as an on-line tool to aid understanding of the changes.
Finally, as terahertz technique can probe coating integrity and thickness, as well as interface between the coating and the substrate; it can be used to monitor these quantities during product development, process scale-up and manufacture, as well as in root cause troubleshooting for current operations.
References
1) Barnes K. Reformulation trend capturing pharma. In-Pharmatechnologist.com. January 2007.
2) Spencer JA et al. Delayed release tablet dissolution related to coating thickness by terahertz pulsed image mapping. J. Pharmaceutical Sciences. DOI 10.1002/jps
3) Taday. P.F. Terahertz Pulsed Imaging for nondestructive testing of pharmaceutical products. Spectroscopy. 24(4) 2009 28-36.
4) Ho L et al. Analysis of sustained release tablet fil coats using terahertz pulsed imaging. Journal of controlled release. 119 (2007) 253-261.
5) Ho L et al.. Applications of terahertz pulsed imaging to sustained-release tablet film
coating quality assessment and dissolution performance. Journal of controlled release. 127 (2008) 79-87.
6) Ho L et al.. Monitoring the film coating unit operation and predicting drug dissolution using terahertz pulsed imaging. Journal of Pharmaceutical Sciences. Published online 14 April 2009.