Lean Materials Management: Lessons from Lonza
As its site in Hopkinton, Massachusetts, continued to grow, Lonza Biologics needed to scale up several key business processes, which had been designed for lower volumes. In some cases, processes had to be completely redesigned. In others, the company opted to use Lean and Operational Excellence tools to wrest the most value from its existing operations.
Lonza began the process of implementing Lean in its facility by focusing on materials management, because it is a key front-end process that drives downstream performance in many areas. This article discusses how the company went about the process, and what it learned.
Improving materials management was critical because the capacity-constrained site had already experienced problems. Out of stock conditions, for example, were caused by a variety of issues: inaccurate inventory counts in the MRP system; inability to rapidly move materials through the receipt and testing processes; use of materials intended for other customers/campaigns (without backfilling); and the frequent need for materials on an emergency basis.
The situation made it difficult to plan and schedule suites and resources. In addition, campaigns were delayed, costs rose, and frustrations increased within and between operating departments.
Project Objectives, Scope, and Timing
Improving Lonza Biologics’ materials management process was jointly carried out by Lonza and Maxiom Consulting Group. It took approximately three months and targeted the following objectives:
- Developing a common understanding of the “As Is” Materials Management process for direct materials
- Identifying and prioritizing process improvements
- Developing and implementing 60-day action proposals to establish high-priority improvements
The scope included all materials management-related business processes beginning with “a stable bill of materials available for production” and ending with “material issue to manufacturing and the decrementing of inventory from SAP [the site’s ERP system].” This included all sub-processes involved in material receipt, quality control, storage, distribution to manufacturing, and disposition.
Project Approach
Three-Day Innovation Workshop
The project began by chartering a cross-functional team of Lonza Biologics’ employees to participate in a Three-day Innovation Workshop—which included elements of education and process analysis and ended with development of action proposals for implementing improvements. Then, during a 60-Day Implementation Sprint, the proposals were addressed.
Lean and Six Sigma tools were utilized within the DMAIC structure to understand the current state and develop the future state. The three days were rigorously structured to bring the team to well-defined action plans for materials management improvements.
60-Day Implementation Sprint
The innovation workshop was followed immediately by a 60-Day Implementation Sprint, structured to ensure that the improvements identified in the workshop were actually made and benefits were realized. As a vehicle for implementing improvements, the Sprint was designed to ensure a few key requirements were met:
- maintain the momentum/enthusiasm built during the Workshop
- identify and remove obstacles impeding implementation, and keep stakeholders and team members actively engaged.
In addition to implementing improvements during the 60-Day Implementation Sprint, the action teams developed control plans to ensure the process and behavior changes were sustained and built upon in the weeks and months to follow.
The same Lonza Biologics employees that participated in the Workshop continued as team members for the Sprint, supplemented by other ad hoc resources with additional subject matter expertise.
Workshop Activities and Outcomes
During the Three-Day Workshop, which initiated the project, the Lonza Biologics team went from a standing start to defining improvements to be implemented during the Implementation Sprint. Key activities performed during the Workshop included:
- Defining and mapping the process inputs, outputs, suppliers, customers, activities, and roles
- Conducting “value-added” versus “hidden factory waste” analysis of process activities
- Assessing current process performance in the areas of cycle time and labor utilized
- Identifying key process stakeholders , their requirements, and barriers to improved performance
- Developing a vision for an improved Materials Management process
- Applying Lean techniques to designing improvements to the process
- Sorting and prioritizing the best improvement ideas to move forward
- Developing a set of action proposals for the high priority improvement ideas
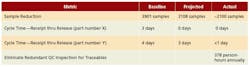
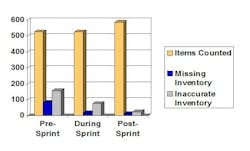
Figure 1 provides an example of one of the outputs developed during the Workshop. In assessing current performance, the team identified dramatic opportunity if wastes could be eliminated.
By the end of Workshop Day 3, the team had developed two detailed action proposals for high-priority improvements to be pursued in the Implementation Sprint:
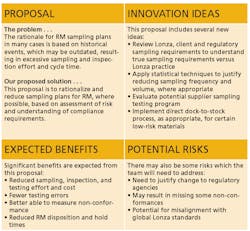
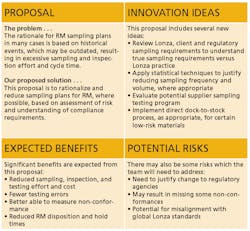
- Review and Reduce Sampling Requirements. This would be focused on rationalizing and reducing sampling plans for raw materials, where possible, based on risk assessment and understanding of compliance requirements.
- Better Utilize SAP within the Materials Management Process. This would enable on-time SAP raw materials transactions and provide QA and QC access to existing SAP capabilities.
Each of the two action proposals was developed utilizing a standard two-page template to ensure consistency for the content and look and feel of the proposals (Figure 2).
Implementation Sprint Accomplishment and Results
Each of the two action teams posted numerous accomplishments and tangible results during the 60-Day Implementation Sprint phase of the project.
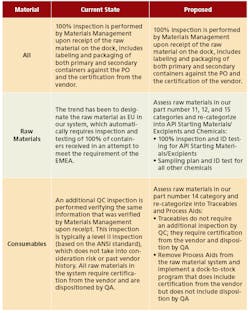
Rationalize and Reduce Sampling Requirements: This action team had numerous key accomplishments during the Sprint:
- Developed a summary of materials received and dispositioned over the past 12 months, including determination of high frequency materials, lot sizes, and key disposition information
- Developed raw material categories utilizing technical input from Manufacturing and Manufacturing Technical Services
- Modified RMS template to align with program changes
- Revised 183 RMS’s (pending implementation of SOP’s)
- Created drafts of six SOP’s that govern the system (Material Control, QC, and QA)
A key element responsible for success was developing and aligning on a revised sampling plan framework that still ensured compliance (Figure 3). In the end, the Rationalize and Reduce Sampling Requirements team achieved or bettered all the improvement targets it initially set for itself (Figure 4).
Better Utilize SAP within the Materials Management Process: This action team primarily focused energy on strengthening skills training and establishing clear expectations regarding the usage of the SAP ERP system that was initially implemented a few months prior. Frankly, this team did a good deal of the work that was not done thoroughly during the initial SAP implementation.
Key accomplishments during the 60-Day Sprint included:
- Strengthened and, in some cases, developed required materials for SAP training
- Set up a training facility and began implementation of a training program for new users to help bolster the ranks of trained people
- Conducted a baseline physical inventory for all suites to ensure SAP inventories were correct
- Trained 9 new SAP users (bringing the total number of users to 18)
- Facilitated four stakeholder meetings and obtained stakeholder commitments to improved frequency and rigor of SAP use by Manufacturing
Lessons Learned and Conclusion
At the conclusion of the Implementation Sprint, the Lonza Biologics team reflected on the process used and results achieved during the project. Three major themes emerged as key to their success and as items they agreed need to be addressed in any subsequent major improvement work:
Active involvement of site leadership is critical to implementing and sustaining change. Leadership involvement demonstrates the importance of the initiative and provides for timely addressing of the inevitable major obstacles that are encountered throughout a major improvement project. Additionally, site leadership involvement reinforces the “lead by example” element of change which is very visible and powerful.
Proper resources must be available. Time availability to participate by those who know and have a stake in the processes is critical. Major change cannot be addressed as a small “side-effort;” it requires resources with significant time and energy to dedicate.
During the improvement process, all avenues/possibilities must be open to consideration. This helps ensure significant change occurs, not just small, incremental change. Ensuring that the team members understand that they are basically unconstrained in their thinking also supports the development of innovative, fresh solutions that tend to revitalize the operation.
Overall, the site was very pleased with the improvements made to the business processes as well as the operational excellence skills/knowledge assimilated by the organization. Since the completion of this project, the site has made great strides in formalizing its Operational Excellence program and is continuing its improvement journey.
About the Author
Fred Greulich is Director of Operational Excellence for Maxiom Group. Jason Martin is Quality Control Manager for Lonza Biologics Inc. in Hopkinton, Mass.