Manufacturer X wants to increase production capacity. Senior management gets on board and hires a Lean Six Sigma expert to conduct a Value Stream Analysis to identify areas where there is production waste. Shop-floor personnel are trained in fundamental tools of Lean production and begin the task of rooting out waste and increasing efficiency.
It’s a typical scenario, says Tim Whitmore, Vice President of Simpler Consulting, specialists in Toyota and Lean methodologies. And yet absent from the process are middle managers, who play the role of casual bystanders. Whitmore refers to these people as the “frozen middle.”
Bryan Winship, Principal of Tunnell Consulting, calls this the “black hole effect” (see box below). Top leaders of the company sponsor the initiative. But the initiative is implemented at the grass-roots level. Middle management is left in the void.
No matter what metaphor you choose, when things go wrong with OpEx, middle managers often take the blame (Figure 7). There’s irony here. The whole idea of operational excellence initiatives is not to lay blame but to focus on opportunities and how such initiatives can empower all parties and serve as opportunities for cross-functional communication and camaraderie.
“There’s a need to more fully engage all stakeholders,” says Winship.
Chalk it up to immaturity. Operational excellence as a formal process is still in its nascent period within the pharmaceutical industry.
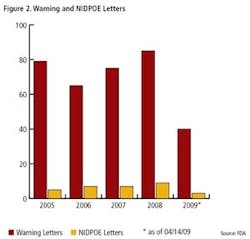
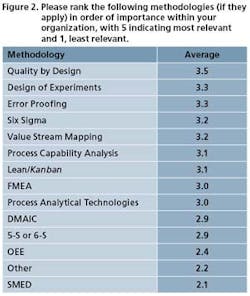
Pharmaceutical Manufacturing’s 2009 Operational Excellence survey reveals a gradual increase in implementation of key tools and methodologies of Lean Six Sigma (and regulation-driven practices such as Quality by Design) over previous years (Figures 1 and 2), but most experts agree there is much development still to occur.
As the era of high margins and low competition has disappeared, “there’s been a heightened awareness of the importance of operational excellence in pharma” says Pascal Dennis of Lean Partnerships, Inc., and author of “Getting the Right Things Done: A Leader’s Guide to Planning and Execution” (available at www.lean.org). Still, says Dennis, “it’s early in the game for pharma companies” and as a result, operational excellence programs are bound to experience growing pains.
One such pain, ironically, might be Quality by Design. As Figure 2 suggests, Quality by Design is becoming the major OpEx priority of many companies. (This has not been so apparent in our previous surveys.) Not surprising, since it is being actively promulgated by FDA. A conundrum arises. With QbD such a focus of drug manufacturers’ efforts in 2009, will other operational excellence initiatives get pushed aside, though they may overlap or complement QbD? More on this to follow.
A Hole in the Middle?
No matter how well-conceived operational excellence programs may be, some will struggle. As Figures 3 and 4 indicate, a good segment of companies pay lip service to operational excellence and can be preoccupied with fire fighting or the bottom line.
As Figure 7 suggests, upper management as well as regulators (and to a lesser degree line operators) can hinder success. But right up there as well is middle management.
“Middle managers are not the problem,” Dennis says. They’re often accused of dragging their feet and causing bureaucracy, but this is more often a failure of top management to clearly articulate objectives related to operational excellence. “What is required is communication rather than scapegoating,” he says.
Figure 1 illustrates some of the key organizational objectives at your companies. They’re admirable goals, but will work only if they have C-level support. “If something is not a companywide initiative with strong executive sponsorship, it’s like a car with little gas,” says Arvindh Balakrishnan, Oracles’ VP of its life sciences business unit. “It takes a level of executive sponsorship that middle management cannot provide.”
If upper management fails in its responsibility to fully support an initiative, or lacks competence in communicating it and involving the rest of the organization, the black hole is inevitable. Middle management, plant and lab professionals, administrators, and everyone else in the organization will be caught in the vortex.
Management by Objectives (a term coined by Peter Drucker) is one way to avoid this pitfall, Balakrishnan says. MBO is a process whereby management and employees agree upon corporate objectives. “Invariably, middle management will have significant operational issues ongoing,” says Balakrishnan. Unless there’s a mechanism in place such as an MBO, then middle management will not be fully on board. “If they’re judged only by their own operational metrics, that’s what they’ll focus on.”
What can middle managers do to make sure they’re fully engaged and relevant? Think cross-functionally, Balakrishnan advises. If you’re focusing on Lean packaging, make sure the manufacturing manager, production scheduler, purchaser, and other key personnel are involved. “Put together a cross-functional team around the same problem,” he says.
As Figure 8 attests, a majority of pharma companies may still be considered fair or poor when it comes to cross-functionality. Project metrics can also help by making sure that there is some way to define progress and success. This doesn’t have to mean investing in some sophisticated software program, Balakrishnan says.
“It could just be one admin that is keeping track of things.”
And make sure that middle managers are measured cross-functionally, says Whitmore. If middle managers are measured only by their own function’s performance, they have little incentive to think cross-functionally. Companies must measure the performance of the Value Stream as it’s being improved, he says, in terms of growth, costs, safety, on-time delivery, etc. “Thus, the middle manager now has a set of metrics that are independent of his function,” Whitmore says.
Biogen Idec’s senior manager for Operational Excellence, Rui Coelho, agrees that top management must drive operational excellence. At Biogen, it was a senior VP who drove initial interest in Lean and persuaded the organization to get on board. Nevertheless, that doesn’t mean Lean thinking can’t start lower in the organization, and have a positive impact.
Middle managers with Lean or Six Sigma experience are a must, he says. “The more they’ve lived it in a prior life, the more they understand and can further it.”
Aside from that, Coelho sees success in those individuals who have project management and analytical skills, scientific and subject matter expertise. And project leaders need to be given assignments commensurate to their abilities. “These aren’t usually high-level, Six Sigma statistical needs,” he says. Biogen has gotten good traction with projects such as error-proofing, DMAIC, and other methodologies that are easily coachable and easily learned.
Training, or rather cross-training, is critical to middle management’s success. Coelho refers to Figure 1 in our survey, which shows “Improve manufacturing agility” as the top goal among respondents to the survey. Agility, if it’s going to happen, must be reflected in the workforce. For this reason, Biogen Idec has a program of rotating employees so that they experience multiple positions and departments.
Dennis is a believer in the Japanese concept of yokoten, roughly translated as “lateral learning” or the lateral transmission of knowhow within an organization, as a means of leveling the responsibility of operational excellence throughout the organization.
The term “black hole” has been used by change management professionals for many years to describe the change resistance that occurs when there are project sponsorship gaps, says Bryan Winship of Tunnell Consulting. In astronomy, a black hole refers to a vast region of space whose pull is so strong it sucks the energy from everything around it. Black holes in an organization may be invisibly sucking the energy from operational excellence efforts, reducing their impact and lengthening their timeline.
The black hole effect is created when the operational excellence project sponsor, the person with the organizational muscle to clear roadblocks and drive change, is organizationally removed from the person leading the project, Winship explains. This creates commitments gaps that foster resistance and create a commitment black hole in the organization.
The black hole effect occurs even with small divisional or departmental projects. For example, if the project was being sponsored by the Quality Director as an improvement to the quality operation, a black hole may be created at the quality manager/supervisor level. If the project team comprised people from other parts of the organization, then a black hole may be created at the manager/supervisor level of their respective organizations, creating resistance to team participation.
Finally, if they are to be heard, middle managers must be aggressive. “Middle managers need to study and absorb the basics of Lean,” says Dennis. “That takes humility and tenacity.”
Pass It Along: Vendors and Suppliers
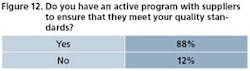
As OpEx matures in the pharmaceutical industry, vendors and supply partners are expected to understand and participate in critical programs. In some cases, they’re expected to have their own programs. Figures 11 through 13 illustrate the degree to which drug manufacturers are including vendors and suppliers in their operational excellence thinking.
But as Figure 10 indicates, roughly three-quarters of our survey respondents see vendor variability as a major or at least significant problem. (This is up roughly 10 percent from last year’s survey numbers.)
Could there be a divide between reality and perception? Could vendor partners be performing better than we give them credit for? Balakrishnan recalls reading an article that suggested that companies pay suppliers to reduce costs. This is the kind of thinking that pharma needs, he says. “We can’t have a standoffish attitude toward trading partners,” he says.
Vendors and suppliers need to be true partners, extensions of an organization. They can be included in engineering and design, process development, or tech transfer, and collaborate on process improvement in whatever area they are involved. “Put mechanisms in place so that they are incented as you are,” says Balakrishnan. “It’s a mindshift.”
Dennis agrees. The Lean business system is a way of thinking, he says. To internalize ideas about waste, flow, and pull, suppliers must learn by experiencing so that they learn in a “visceral” way.
Coelho sees extending operational excellence programs to outsourcing and supplier relationships as the next big step at Biogen Idec. It is not something that has been a primary focus as of yet, but will be more and more, he says.
Pass It Along: The Whole Organization
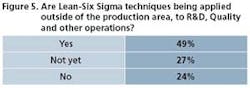
Operational excellence programs have established traction in commercial manufacturing. They’re also broadening to include all tentacles of the organization, as Figures 5 and 6 attest. R&D is one area. “Research and development has always been a creativity-driven organization,” says Balakrishnan. “They value independent thinking, so getting them to follow certain processes has always been a challenge.” One good sign: QbD ideas and efforts are moving from commercial manufacturing to development, where it rightfully should start, he says.
OpEx has taken root in many areas at Biogen Idec, says Coelho. Biogen’s formal, corporatewide operational excellence initiative is about two years old now, he says, and has steadily infiltrated all parts of the organization. As the company’s clinical trials have increased (now to 60-80 per year), opportunities for Lean in the supply chain have proliferated. Initiatives begun in commercial manufacturing have trickled down into development, he says. (They’re still behind similar programs in manufacturing, he says, but have matured to where they’re maybe “six months behind” in terms of their depth and teams’ abilities to implement them.) Even senior financial management has gotten on board—following some Kaizen events, accounting books are closed much more rapidly now than they were before.
“Once you create pull in one part of the organization, then other people will watch and want to come on board,” Coelho says.
And many areas are ripe for change. “When the pain of doing things is greater than the pain of changing,” that’s when groups begin looking around for solutions, he says. If you make operational excellence visible within the organization, it will pull those groups that are ready into its net. Some departments aren’t ready, Coelho says, and if you push Op Ex upon them, it can backfire.
Biogen operational excellence projects can be driven at the department, site, or global level, Coelho says. Each level’s projects are guided by steering committees, and of course global projects take priority over others.
Is QbD Crowding Out?
As Figure 9 shows, Quality by Design has come to dominate many companies’ operational excellence efforts. Wonderful. FDA no doubt would be pleased, and those organizations that have leveraged QbD most likely have positive results to share.
But even complementary programs—e.g, Lean and Quality by Design—can compete for time and resources. So could it be that QbD is crowding out other initiatives—as Figure 9 also suggests?
“QbD is the hottest topic right now, and that’s mostly driven by FDA,” says Winship of Tunnell. Nobody’s treating QbD as optional, which means it gets elevated status by default. “There’s less of an impetus or drive to implement [Lean, Six Sigma, and other initiatives], even though they’re complementary to QbD—because there’s a regulatory need for QbD.”
The problem, says Winship, is that manufacturers often see QbD, Lean, etc. as time-consuming preoccupations. Sure, there is a great deal of training and administration involved in getting ramped up, but these drains can be offset by gains. “I’m happy that QbD is a regulatory requirement now, but it’s not something that takes extra time or extra work,” he says. And that’s true of other operational excellence programs. “Getting it right the first time, with a structured methodology, is not any harder than not getting it right the first time.”
“Speed to market is everything and oftentimes overrules all other decisions,” Winship continues. “We don’t often have time and effort to implement QbD, Design for Six Sigma early on in processes. We have to do that after the product is on the market, and we miss an opportunity.”
Compliance shouldn’t dictate priorities. “Don’t suck all the air out of your other initiatives,” Balakrishnan warns. “Any initiative that has the word quality in it will rise to the top of the heap in pharma . . . but you have to put other initiatives on equal footing.”
Decide what your objectives are and stay the course, Pascal Dennis reminds. Lean consultants are prone to sound bites, but Dennis offers a quick bit of advice that’s too good to pass up: “The main thing is to keep the main thing the main thing.”