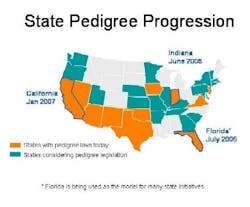
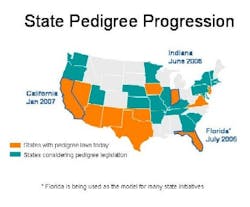
With jurisdiction over drug distribution, state laws require drug pedigrees which provide a certifiable transaction history for each product. Eleven states have pedigree laws, and more than 20 states are considering similar legislation (see color-coded map below). Florida, Indiana and California set the pace with fast-approaching deadlines: Indiana, June 2006; Florida, July 2006; and California, January 2007. Clearly pedigree is an important anticounterfeit and antidiversion layer of protection whose time has come.
Protecting their residents from counterfeits, Florida and California require all prescription drugs to be tracked from the pharmaceutical manufacturer through each wholesale distributor to the pharmacy. California requires that the pharmaceutical manufacturer initiate the pedigree record. Taking a slightly different approach, Florida requires each pharmaceutical company to either start the pedigree record or respond to all downstream trading partners who need to authenticate the first transaction between the pharma company and the first wholesaler.
The minor variations between the state pedigree requirements pose no problems because the EPCglobal Pedigree Messaging Format finalized earlier this year addresses the requirements across all the states. More than half a dozen software vendors now offer self-authenticating electronic pedigree software to streamline the pedigree authentication and verification processes.
None of the states require RFID as a prerequisite for pedigree. Electronic pedigree is an important layer of anticounterfeit protection which can be implemented now. E-pedigree solutions have been designed to work with RFID when drugs are tagged, but dont expect all drugs to be uniquely identified with a serial number.