CONSIDER THESE STATISTICS:
1. Drug recalls have grown at an alarming rate. The last three years have seen almost as many recalls as the previous nine years combined. Contamination during the manufacturing process being one of the biggest contributors.
2. $50 billion/year losses in manufacturing costs from inefficient processes.
3. According to the FDA, ~300 drugs are currently in short supply.
The pharmaceutical manufacturing industry has remained with batch processes, backed by rigorous testing at each stage (Figure 1), for well over 50 years, especially when it comes to the preparation of solid oral dosage forms. It has relied heavily on standard operating procedures and process control without being able to solve the fundamental issue — variability in end-point of a typical batch process that can only be controlled by the subjective intervention of a human operator. Statistics, like the ones listed above, indicate that pharmaceutical manufacturing cannot afford to have any controls that are subjective and variable.
Continuous processing could lend itself to solve the end-point variability and free the “process” from operator dependence. That said, continuous process can be exploited by the pharma industry if and only if certain prerequisites are maintained.
Continuous manufacturing practiced in several industries (not related to pharmaceuticals) is typically associated with wide Residence Time Distribution (RTD) due to back flow and stagnation, with inherent risk of quality and control. As such, continuous manufacturing is employed to achieve economics — as in gourmet vs. mass production of food products.
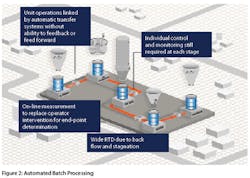
In the pharmaceutical industry, some manufacturers employ highly automated batch processing in lieu of continuous manufacturing considering it as a superior option. In such instances, there continues to be multiple batch operations such as fluid bed drying linked via automatic transfer systems with these unit operations still needing individual control and monitoring at each stage (Figure 2). Operator intervention is tried to be replaced by on-line measurements for determination of end-point. However, neither automated batch processing nor wide RTD continuous manufacturing or a combination of the two is suitable for wide-scale adoption in the field of pharmaceutical manufacturing. The high degree of control required for maintaining product quality can only be realized in a steady state and in the event control is lost, the ability to deal intelligently with the product in stream has severely restricted the application of continuous processing in the pharmaceutical industry.
In other words, the pharmaceutical industry needs Next Generation (21st Century) true continuous manufacturing that has certain unique attributes that satisfy all of the requirements — regulatory and quality, among others.
A PARADIGM SHIFT: TRUE CONTINUOUS, SINGLE POT MANUFACTURING
True continuous, single pot manufacturing is built on the principle of a “flow stream in continuity.” Utilizing a twin screw processor, that has a unique ability to clean itself, true continuous manufacturing relies on keeping the input, process material and output in a continuous flow, with particles moving as a highly stratified process stream.
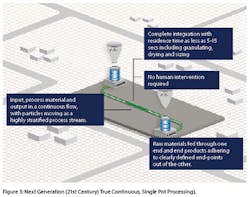
The energy transfer is made effective using thermal, mechanical or chemical means at wide-ranging magnitudes with minimal shear or pressure peaks providing specific advantages in handling sensitive input materials that may or may not rely on the use of water or vapor. Granulation process can typically be achieved with residence time as little as 5-15 seconds (including granulating, drying and sizing), and the optimized granules obtained are ready to be compressed into tablets or filled into capsules (Figure 3).
True continuous, single pot processing provides significant improvement in building both temporal and spatial control in process engineering through removal of hot spots and dead zones, while maintaining higher level of process continuity. Another key aspect is the principle of integration. All operations are integrated into a single piece of equipment, hence the term ‘single-pot,’ minimizing the need for human intervention. Raw materials are fed through one end and end products adhering to clearly defined end-points come out of the other.
Moreover, true continuous processing allows for a significantly smaller footprint (due to integration), is flexible and versatile, and can significantly reduce capital costs and wastage besides minimizing the burden on the environment.
IT'S TIME TO CHANGE
- Elimination of hot spots and dead zones
- Traceability (stratified continuous stream)
- The ability to maintain steady state of control
- Near complete delivery
- Versatility (easily adaptable to meet differentiated needs during process development)
- Quality by Design (well established risk and product quality)
- Economical development
- Ease of scalability (linear and non-linear)
- No metal dust contamination
The FDA is, in fact, urging manufacturers to shift to continuous processing for its numerous advantages, including flexibility, reliability and scalability. But above all, the pharmaceutical manufacturing industry faces a dire need to reduce its burden and shift its focus onto quality manufacturing (QbD via well-established risk and product quality). True continuous processing not only addresses the most fundamental concerns relating to regulatory requirements and quality, but also provides manufacturers with a significant reduction in capital and operating costs, manufacturing footprint, wastage and downtime, while significantly increasing the speed to market.
About the Author
Babu Padmanabhan, Ph.D., has made significant contributions to the field of polymer processing. His work in designing processing section components has led to the development of intelligent compounding technology that has redefined pharmaceutical manufacturing. The fractional lobed processor invented by him provides critical break-through in enabling novel true continuous technologies and solutions. For more information about STEERLife, visit www.steerlife.com.
REFERENCES
Number of Drug Recalls Surges at FDA, Led by Mid-Level Concerns, Posted 11 August 2014, by Alexander Gaffney, RAC
Nickerson, J.; Macher, J., Pharmaceutical industry wastes $50 billion a year due to inefficient manufacturing. McDonough School of Business, Georgetown University, and Olin School of Business, Washington University
FDA Drug Shortages
Drug Shortage in the U.S. May Pose Deadly Problem for Patients
FDA Perspective on Continuous Manufacturing, IFPAC Annual Meeting, January 2012