Biopharma researchers and manufacturers, and the companies that supply their production systems and materials, are paying greater attention to improving the major stages of upstream, downstream, and fill and finish production steps as the global market for biologics continues to expand.
Despite significant advances made in recent years to increase biologic yields, as well as the intensity and consistency of these processes, there is still the potential for innovation in this area in order to reach more efficient production of therapeutic proteins. Finding new approaches to improve protein expression; developing more flexible, cost-effective and robust manufacturing processes that lead to higher yields; and solving the complex task of refolding proteins into their active state are among the challenges faced by the industry.
As cell culture processes are influenced by nutrients and trace elemental impurities, cell expansion steps requiring various media components can impact the overall process and result in a lower final product yield, higher amounts of product-related impurities and/or longer process time. With a better understanding of the properties of these media and supplements, process scientists can take steps to better manage the quality and consistency of their cell culture raw materials in order to minimize variability in the upstream process.
From inoculum to bioreactor production
The upstream process steps for producing a biopharmaceutical from vial to full-scale bioreactor production are well-established. The workflow starts with a small cell culture, changing the cells’ genome, then scaling up the culture in a series of steps to produce desired quantities of the target biologic. Although operating conditions may vary from company to company, and may depend on molecule-specific processes, the basic steps of cell expansion involving growth of the target cells from a vial into an initial stage bioreactor are performed to keep the cells close together through the first growth, or expansion, phase.
Throughout the expansion stage, it is crucial to exercise as much control as possible over all cell culture conditions to assure healthy, growing cells and protein expression in line with the process design. This includes exercising control over the conditions within each bioreactor, including the temperature and presence of key gases (e.g., oxygen and carbon dioxide). The composition, purity and stability of all the cell culture media — including carbohydrates, like glucose, amino acids and supplemental vitamins — should also be considered.
If the conditions and materials are not properly understood and controlled during this process, the cell culture will start to die or may fail to expand enough to move to the next step. This requires the process to be stopped, the materials to be disposed of and the process to be started over with initial cell inoculum.
Expression systems
Although several expression systems exist, bacterial or microbial systems, yeast and mammalian cell cultures are the three most common.
When biopharmaceutical drug production was first scaled up in the 1980s, Escherichia coli was the most widely used expression system. After that, the yeast Saccharomyces cerevisiae was also adopted by biopharma manufacturers. Insect cells were explored and used as a method to produce vaccine-oriented proteins, but mammalian cell lines have become the most widely used cell systems due to their ability to produce the glycosylated proteins necessary for these processes.
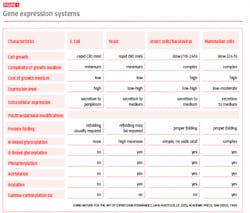
A comparison of key features of the major expression systems (Fig. 1) highlights factors that are critical to managing the efficiency and improving the optimization of cell expression systems. Two of the most important controlling factors when comparing systems are the rate of growth and the complexity of the cell medium.
Microbial systems have relatively fast growth rates, less complex growth medium and a lower cost of materials. Mammalian systems, on the other hand, are much more complex: these cells undergo several complex cellular processes (such as N-linked and O-linked glycosylation, acylation and gamma-carbohydration), which unfold over several days and can be difficult to control. If there is a problem with the expression system and the process is not on target, then less time is wasted when stopping microbial systems during the expression process, cleaning all materials and restarting as compared to the mammalian cell culture method.
Glycosylation is difficult to control in mammalian cells since it’s dependent on multiple factors, from clonal variations and cell culture conditions to media components and materials used in cell expression steps (carbohydrates and pH control substances such as sodium bicarbonate). Optimizing glycosylation is more difficult because cell culture media is line dependent. Different cell lines have different metabolism and nutrient-consumption requirements. It has been demonstrated that trace elements in cell culture media, which can be introduced via materials like carbohydrates or supplements, can cause different reactions that affect cell expression yields.
In addition, if the protein being manufactured has reactive spaces that respond to trace metals or ratios of certain metals, there can be reactions during protein folding and unfolding such as oxidation. If enzyme therapy replacement biologics are being produced, the enzyme can have trace metals as part of their intake which can impact production yields.
Managing trace elements in cell culture media
The impact of trace elements in cell culture media on the expansion and bioreactor steps in upstream processing has become a significant area of interest for many biologics manufacturers. Current industry practices use standard basal cell culture media as a starting point. In many cases, media developed by commercial providers and optimized for specific cell lines is sourced; however, in special circumstances, biologics manufacturers may develop proprietary cell culture media for specific process requirements in order to ensure optimal performance. In either case, if the components used in the media manufacturing process are not as fully characterized as they could be, this may cause the basal culture to contain levels of trace metals (e.g., magnesium) that could interfere with protein folding and unfolding at the expression stage, leading to a shortfall in expression yields or contamination of the resulting protein.
It has been demonstrated that trace metal concentrations as low as parts per billion can impact protein glycoforms. One study showed that manganese impurities present in a range of ferrous sulfate lots (used as a supplement) generated different glycosylation profiles in monoclonal antibodies. Another study examined the effects of iron and manganese on protein glycosylation and found that parts-per-million concentrations of iron altered protein glycosylation while manganese altered protein glycoforms at concentrations as low as 20 parts per billion (ppb).
The complexities of trace metal impacts can multiply once other elements are added to the basal culture media during various expression steps. For example, a process could call for the addition of one nanomolar of manganese at a certain step to aid in protein expression. In some cases, the process engineers will try to increase the supplement to 10 nanomolars, but the results lead to negative impacts on cell quality and yield. The process then reverts to the prior one-nanomolar supplement. If the cell culture components have not been fully characterized in the creation of the basal media when there is already a significant level of manganese present, then adding the one-nanomolar supplement could have the same negative outcome.
Given such complex issues, there should be significant potential to improve the performance of expression systems by improving the characterization of carbohydrates, amino acids and other cell culture media components, given how they transition through different-sized systems.
A significant step forward is to use cell culture media and supplements produced under cGMP processes with fully characterized trace metal levels. Ensuring that trace metal levels are consistent from lot to lot is one method to help reduce and even eliminate sources of variation in upstream processes. Completely eliminating trace metals from cell culture media, however, is not the goal; rather, tight control of metals at established ppb levels for a given expression system can contribute to improving outcomes. Studies have shown that trace metals, such as copper and manganese, can aid in glycosylation and other protein expression processes.
It is also vital for researchers and manufacturers to establish accurate, reliable levels of trace metals for their processes and cell lines, using design of experiment (DOE) studies to fully characterize materials. The results of these studies can accurately establish the appropriate trace metal levels for each cell line and cell expansion process. Biologics manufacturers then need to ensure that materials used in the biologics production process conform to these established levels, and thus enable producers to keep upstream yields on target.
Establishing this level of control also depends on having a better understanding of the data generated by expression and production system processes. Leading biologics manufacturers are expanding the use of data mining and AI-based analytics technologies in their operations to achieve this control. This movement is applying many of the same techniques from “Industry 4.0” manufacturing initiatives. The goal is to use the wealth of data generated from production processes to gain deep insights into factors that govern process performance, enabling a vision for a biologic “factory of the future.”
Referred to as “Biopharma 4.0,” a biologic “factory of the future” will see more IoT-connected “smart” devices installed in facilities. These devices, along with their connected software platforms, will let plant operators use real-time data generated by their processes to maintain and improve the performance of their production systems. Biopharma 4.0 also envisions platforms that are powered by artificial intelligence (AI) that will be able to predict and maintain optimal operating conditions autonomously. This improved data insight and control over processes will enable greater consistency and reduced risk of batch failure.
Over time, the goal is to create a perfect, or “golden,” batch — an outcome that requires statistically relevant data from process steps and all the raw materials used in those processes. As a result, strong collaboration between suppliers and manufacturers is required to improve data collection and access. Suppliers play a role in proactively supplying data for predictive models, setting up automatic eData transfer mechanisms and enhancing analytics capabilities to further streamline the process.
Combining data-driven insight with fully characterized materials produced under cGMP conditions will allow for new levels of process control and the possibility of significantly increased upstream yields to be achieved. In the case of cell culture media, implementing state-of-the-art analytics can help manufacturers understand how variations present in media components, along with aspects of expression and bioreactor systems, can be correlated in a more meaningful way with variations in expression system results. Various models such multivariate analysis can be used to understand the process output trends, influence of multiple factors and predict highly complex cell culture process performed over period of weeks.