Connecting labs to power digitalization
A fully integrated network of laboratories can deliver significant benefits to the pharmaceutical industry. By facilitating improved connectivity and collaboration between their network of laboratories and manufacturing plants, pharma companies can become more productive, strengthen their resilience to disruption and offer greater job satisfaction for their scientists. To this end, digital transformation programs are underway in pharmaceutical companies worldwide.
Well-integrated data systems are a critical component of the connected laboratory, providing a single source of truth accessible across the organization. At present, however, data silos and unconnected systems represent a significant hurdle for many pharma manufacturers, limiting their transformation efforts.
Improving data integration is a challenging process. Attempts to address single gaps between instruments, systems or laboratories won’t necessarily deliver data harmonization — and could even hinder future transformation efforts.
By creating a holistic data strategy and implementing advanced data solutions to connect their laboratories to each other, as well as integrating quality data from the manufacturing plant, pharma organizations can overcome data silos in manufacturing and continue their digital transformation journey.
The drive to address data silos
Data management is critical in pharma manufacturing, for both compliance and timely workflow decisions. High volumes of data both from QA/QC laboratories as well as from Enterprise Resource Planning (ERP) systems must be safely and appropriately stored, so that information can be accessed by the right people at the right time.
However, pharma manufacturers often face data silos. A combination of the disparate data formats of lab instruments, isolated software systems, unintegrated workflows and past acquisitions mean that many organizations have a fractured information landscape. Data may be isolated on one system or available to just one group, limiting communication between individual labs, sites, regions and even external collaborators. Information that could be critical to plant managers waiting to release product, or when collated with other details might provide a crucial answer to a manufacturing issue, is often left adrift.
These data silos also pose significant challenges for the manufacturing process by creating gaps in data integrity. This could compromise the completeness or consistency of datasets, and the accuracy of the insights discerned as a result. Equally, disparate systems require the manual transfer and input of data, which can represent a sizeable drain on efficiency, particularly where data needs to be transferred between international sites. The productivity of scientists is therefore reduced by needing to spend time on these highly repetitive and error-prone tasks.
Without an integrated, standardized system to share information between the laboratories and plants, pharma manufacturers may not be able to realize improvements to manufacturing processes and workflows. Meanwhile, at a strategic level, decision-makers may lack a holistic view of the business to inform the choices that will guide the future of the organization.
Accelerating integration
The drive to create fully integrated data systems and strengthen data integrity has accelerated over recent years and even months. Establishing a single data repository improves data tracking, storage and reporting; that in turn enhances efficiency, facilitates scalability and flexibility across the organization, and improves decision-making. There has also been clear support from a regulatory perspective, with the U.S. Food and Drug Administration establishing the ALCOA+ principles for data integrity in pharmaceutical manufacturing, stating that data must be attributable, legible, contemporaneously recorded, original or a true copy, and accurate.
Pharma manufacturers are now more aware of the potential of cloud-based storage and applications to support the delivery of data integration. The cloud helps facilitate a single source of truth for data and enables easier data sharing across teams and global sites. Importantly, pharma manufacturers have growing confidence in data security in the cloud.
More recently, COVID-19 has underlined the need for data management improvements to support resilience in the face of disruption. Laboratories that are heavily dependent on manual data input have seen their operations particularly disrupted by social distancing requirements. This has in turn made it more difficult to provide plant managers with the timely QA/QC data required. Integrated digital processes, together with data automation, can keep labs operational with fewer scientists on site, and maintain a seamless flow of data to manufacturing plants.
It’s also true now more than ever that pharma manufacturers need to be well positioned to provide the fastest and most efficient operation to deliver high quality drug products. This can only be possible if all opportunities are taken to automate and streamline processes, and to ensure information is made available to those who need to make critical operational decisions. Collating QA/QC data from labs with manufacturing data from ERP systems provides a more holistic view of operations throughout the organization, enabling continual improvements to maximize production efficiency.
In addition to significant short-term operational benefits, single data repositories will enable pharma manufacturers to conduct more sophisticated analyses of their laboratory and plant data to identify longer term patterns and opportunities. With a standardized, central data set, pharma manufacturers have the opportunity to benefit from utilizing data analytics, including machine learning, to spot trends over time. These insights enable continual performance improvements, enabling organizations to realize the full value of their data.
The opportunities to maximize the value of data for both operational optimization and longer-term transformation are clear. However, the level of complexity in labs combined with other operational requirements can make delivering integrated data systems a significant challenge.
Establishing a holistic data strategy
The most effective way for pharma manufacturers to establish a fully integrated data system is to develop a holistic data strategy encompassing the whole organization.
In the past, many labs have attempted to address gaps in their data processes individually, with single upgrades or patches that might cover a single integration or section of the organization. However, this approach will leave gaps at the end of the process, and may render it more difficult to integrate new instruments, software or technologies in the future.
By contrast, taking the time to develop a holistic data strategy enables the organization to create a fully connected ecosystem, with a single source of truth from the lab to the boardroom. Manufacturers can map their existing systems, determining where gaps lie and what information needs to be captured and connected to deliver value for the business.
Technology specialists can support this process by providing strategic guidance and identifying the data solutions that will most effectively integrate instruments and software systems, while enabling the adoption of new technologies further down the line.
Integration in action
A data integration program was recently undertaken by a large pharma company. The Chemical and Pharmaceutical Development (CPD) department was using six disconnected systems and over 1,000 disconnected instruments. Importantly, there were large differences between sites and departments, with heterogeneous IT landscapes and inconsistent LIMS usage, which meant the project required significant conceptualization to create one strategy incorporating every system.
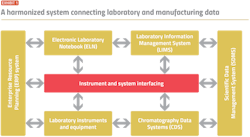
The pharma company worked with a technology partner to improve data harmonization. Laboratory Information Management System (LIMS) software was chosen to manage lab data and procedural workflows, and connect with other enterprise systems, instruments and equipment. The built-in integration solution was used to build cross-connectivity between the LIMS, ELN, CDS and SDMS, as well as the instruments and equipment used in the lab (Exhibit 1).
The higher degree of integration achieved has increased efficiency by 20 percent, with additional improvements to both data quality and integrity. Fewer mistakes are likely through transcription errors, while the labs benefit from the security of a full audit trail, including a risk-based audit system. Information isn’t simply captured as “paper on glass,” but intelligently linked to improve searchability. Despite the different modules and capabilities, the user experience across the labs is nearly seamless — and scientists now have more time for what they are most passionate about: Science.
This project also highlights the importance of cultural change to a successful data strategy. Scientists are often required to focus on operational tasks, so don’t have the time needed to make the transition to new technologies or develop new digital skills. By establishing a holistic strategy that incorporates the people who use the systems, pharma manufacturers can ensure their data integration programs are more effective.
Beyond individual transformation programs, technology vendors have a key role to play in the establishment of a universal data format that will facilitate future integration. Organizations like the Allotrope Foundation and Pistoia Alliance are working to establish standards for common data formats, which will benefit end users going forward.
A platform for transformation
Pharma manufacturers can achieve significant operational advantages by addressing their data silos. An integrated system that connects labs and manufacturing plant quality data across the organization can provide greater efficiency and scalability, facilitate flexibility, and improve decision-making. But importantly, establishing an integrated data system will be a key major step forward for enabling improvements both in the lab and across manufacturing operations to support future transformation. Considering the current need for pharma to be ready to rapidly deliver a large supply of vaccines and treatments, there has never been a more significant time to advance on this digital transformation journey.
With truly connected labs, pharma manufacturers can establish greater collaboration across the business, identify continual organizational improvements and, perhaps most importantly, enable scientists to spend more time on productive, valuable and rewarding tasks. By establishing a holistic data strategy, with the support of a strategic and collaborative technology partner, pharma manufacturers can improve workflows today and be prepared to quickly make adaptations for their needs of the future.