Overcoming obstacles to drug absorption
Low aqueous drug solubility in the pharmaceutical pipelines is a pervasive issue. In particular, rapidly growing therapeutic areas such as oncology, antivirals and anti-inflammatory indications are largely plagued by low solubility.
As a result, a number of enabling technologies have been developed to improve oral drug absorption and bioavailability (BA). Salt formation for solubility enhancement is a common technique used during solid form selection for ionizable compounds. However, many salts form hygroscopic materials that can lead to both physical and chemical stability issues. In addition, many salts do not substantially enhance a poorly soluble compound’s bioperformance because of precipitation of the compound in the presence of food (increased pH), common ions in the stomach or as the pH increases upon transfer into the duodenum.
For the more than 50 percent of the compounds in development that are either not ionizable or suffer from stability issues as a salt form, alternative solubility-enhancing technologies are needed.
Many technologies have been shown to enhance drug BA; however, the most notable commercial products are those that utilize lipid-based technologies, amorphous solid dispersions and micronized crystals. The commercial precedence of these key enabling technologies supports their continued utilization in addressing compounds that are either poorly soluble or poorly soluble and poorly permeable, which may be as much as 90 percent.
Those working in the field will, of course, recognize three important facts:
- The diverse needs of all drug compounds currently in development cannot be addressed by a single enabling technology;
- Development success is more likely if a technology is appropriately matched to the compound properties and product needs;
- Correctly selecting the solubility enhancing technology and predicting clinical outcomes in early-phase programs is critical to avoid re-work/re-formulation in later phases of development.
In many cases, more than one technology can be utilized successfully, and commercial considerations such as desired dosage format (e.g. tablets vs. capsules) can play a decisive role. Additionally, some technologies may suffice for phase 1 studies but not for later-phase clinical studies. Drug developers may benefit from increased awareness and understanding of these considerations and how they can influence efficient technology selection and accelerated pharmaceutical development.
Physicochemical obstacles to oral bioavailability
Physicochemical obstacles to oral drug BA include low aqueous solubility (a thermodynamic property) and a slow rate of dissolution (a kinetic property). Low drug solubility can limit the maximum drug concentration in the small intestine and, therefore, drug absorption. That’s because a high concentration gradient between drug in the intestinal lumen and drug in the intestinal wall is required to drive passive diffusion across the intestinal membrane. A slow dissolution rate is nearly always associated with low drug solubility, and it is compounded in instances where drug surface area and/or diffusion rates are also low.
Low aqueous solubility is a property common to drugs that are Class II and IV of the Biopharmaceutical Classification System (BCS). Factors underpinning the property of low solubility include:
- A high crystal lattice energy (which generally increases with increasing melting temperature of a compound)
- A low energy of aqueous solvation (which generally decreases with increasing Log P value of a compound, i.e., lipophilicity), often referenced as “grease-ball” type compounds
- A combination of both, where the impact of a high crystal energy on solubility is exacerbated by a low solvation energy, often referenced as “brick-dust” type compounds
Through an appreciation of these obstacles, developers may rationalize the principal means by which enabling technologies increase solubility and dissolution rate, namely by either reducing the drug lattice energy, increasing available drug surface area, and/or increasing the energy of solvation.
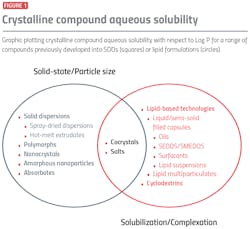
To aid this distinction, Figure 1 provides a simplified separation of key enabling technologies according to the primary mechanisms of solubility/dissolution rate enhancement. For example, nanocrystals can increase dissolution rate by increasing available surface area of drug. Spray drying and hot melt extrusion (HME) solid dispersion approaches are able to increase apparent drug solubility and, therefore, dissolution rate by isolating the higher energy amorphous form. Lipid-based technologies are effective in augmenting drug solubility as dispersed and digested lipid components mix with endogenous bile salt and phospholipid so that it is more favorable to dissolving the drug.
In many cases, however, clear distinctions cannot be made. Some technology approaches have the capability to increase drug solubility through both solid-state and solvation effects.
Biological obstacles to bioavailability
Overcoming the physicochemical obstacles to low absorption may not yield the desired BA. In these cases, biological obstacles to exposure may be apparent and may include:
- Efflux of absorbed drug back into the intestinal lumen
- Pre-systemic drug metabolism in the intestine
- Extensive hepatic first-pass drug metabolism
- Low passive intestinal permeability
Certain enabling technologies may have the capacity to attenuate these biological obstacles to drug BA, particularly by reducing efflux and metabolism in the intestine. Fatty acids and nonionic surfactants commonly used in lipid-based technologies have frequently been shown to inhibit P-gp and BCRP efflux transporters in intestinal cell models or increase transcellular permeability.
Defining the product needs
Besides physicochemical and biopharmaceutical properties of a compound, there are a number of other considerations that typically affect technology selection for a particular application. These considerations include target dose, preferred final dosage form and size, frequency of administration, specific storage and/or packaging requirements, and excipient acceptance. In some cases, these factors play an important part in the technology selection process. It is, however, important to note that many if not all of these “technology restraints” can often be identified prior to the initiation of development work, and such considerations can therefore prove valuable by reducing the risk of pursuing certain technologies that are later deemed to be unsuitable.
From a fundamental understanding of enabling technologies, two additional dimensions may aid in the technology selection process: predictive physiological-based pharmacokinetic (PBPK) models and technology maps.
Firstly, PBPK models predicated on modeling mass-transport are also consulted to ratify technology selection since they can be used to predict pharmacokinetic (PK) performance based on compound and formulation properties. These models are based on the fact that an increase in the concentration of all drug species — dissolved free drug, drug in micelles and various undissolved particulate but “high-energy” forms — will, in turn, increase the extent of intestinal absorption of a poorly water-soluble drug.
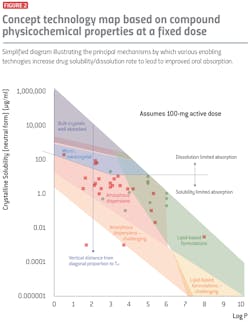
Secondly, conceptual “technology maps” centered on how key physicochemical drug properties impact oral absorption may be useful. One such example of a technology map is illustrated in Figure 2. In this graph, compound solubility in aqueous media is plotted with respect to Log P. The solid diagonal line in this map traces the maximal solubility of the lowest-energy, neutral form of the compound. Decreasing aqueous solubility at a constant Log P value therefore is driven primarily by an increase in the overall solid-state interactions, which is directly proportional to compound melting temperature (Tm).
In the upper region of this map, crystalline solubility is sufficiently high that bioavailability of a 100 mg compound dose is sufficient when using non-enabling formulations. With increasing Log P and/or increasing Tm, however, the decrease in solubility creates the need for enabling technologies to maintain good in vivo performance. Particle size reduction technologies can offer acceptable BA at a 100 mg dose when solubility falls below 1 mg/ml.
However, this tends to result in the dissolution rate failing to maintain the drug concentration at its equilibrium level while it is being absorbed. As the solubility decreases to the range of 10-100 µg/ml (depending on the Log P of the compound), the utility of such technologies will typically diminish. At these low solubilities, it is necessary to utilize technologies that improve drug concentration in the GI lumen above its equilibrium solubility and/or drug transport across the unstirred water layer via sub-micron colloids. Amorphous solid dispersions are highly effective across a broad Log P 0-6 range, but above this range the additional functional excipients provided by lipid technologies are often necessary to solubilize and enhance transport of the compound through the aqueous boundary layer. Lipid technologies also cover a broad Log P 3-10 range, hence there is a progressive overlap region of amorphous and lipid approaches between Log P 3-6 values.
This technology map provides a simplified, two-dimensional insight into how drug physicochemical properties can affect the feasibility and performance of various enabling technologies. However, this map cannot be utilized in isolation as it does not consider other important properties such as biological obstacles to drug absorption. Additional technology maps that evaluate other properties may be used in combination to aid in technology selection.
Conclusions
Drug development remains plagued with low solubility and poorly bioavailable compounds. While numerous technologies have been developed to enhance these compounds’ absorption, three technologies — namely particle size reduction, amorphous solid dispersions and lipid formulations — have emerged as the leading technologies in the field.
By considering the physical and biological factors limiting absorption, the fundamental science behind the technologies and PBPK models, drug developers can identify the appropriate technology to achieve sufficient BA and meet target product profiles. Technology maps, based on historical analyses of key API parameters, can simplify and accelerate technology selection. As the small molecule pipeline continues to contain compounds with low BA, and accelerated development timelines become the norm, a sophisticated understanding of the range of appropriate enabling technologies will become even more important for innovative drug development.