Among the many darlings of pharma press releases, the descriptions pertaining to the industry’s manufacturing facilities are particularly enchanting. “Factory of the future.” “State-of-the art.” “Cutting-edge.” “Next-gen.”
All of these descriptors communicate an underlying desire that characterizes so many pharma facility projects: achieving a state of flexibility.
There are currently over 7,000 active pharma/biopharma construction projects happening around the globe. Companies are spending close to $200 billion to build, expand and update their pharma facilities in the race to get their products into the hands of patients as quickly and safely as possible.1 For decades, the discussion of flexibility has permeated every stage of these pharma facility projects; its praises have been sung by regulators, industry working groups, engineers, solutions providers and of course, pharma marketing teams.
“Everyone talks about getting to that magical place where you can keep the same facility and equipment but just change out the product. This is an admirable goal but it is a long journey taken mostly with baby steps and the occasional hop with a change in technology,” says Bill Brydges, reflecting on his 40-year career in engineering and construction.
But once we pull back the curtain, what exactly does this “flexvana” entail?
Ultimately, a flexible facility is one that is agile enough to adapt to change — whether it be a shift in capacity needs, regulatory demands, manufacturing processes, technologies, products or some combination thereof.
And pharma is no stranger to changing circumstances: An unexpected regulatory rejection, the availability of new or better technology, a crucial piece of equipment that’s been backordered, a specific raw material that is suddenly in high demand, or even a global pandemic will test a facility’s ability to adapt.
While this sought-after state of flex may seem blissful, anyone in the industry who has been involved with constructing or expanding a pharma facility will tell you the road to get there is anything but. It can be gritty, frustrating, expensive — and paved with uncertainties. And even then, the completed project is still likely to fall slightly short of euphoric flex.
But the good news is that flexibility isn’t elusive, and the more pharma can refine the drivers behind their respective flexibility journeys, the more clarity the industry will have in terms of how to get there.
The evolution of flexibility
While the pharma industry can be credited with many life-changing discoveries, it did not invent flexibility in manufacturing.
Similar to quality, flexibility in manufacturing emerged as a strategic imperative.2 In the 1970s, facing increased global competition from world markets and changing consumer demands, American manufacturers began to move away from the Ford method of mass producing standardized goods. By the ‘80s, consumers were more sophisticated and niche, and manufacturers had to incorporate flexibility into their factories and processes to compete.
In pharma, discussions about flexible facilities, mainly centering around the desire to make multiple products in one facility, started picking up speed in the ‘90s. Many credit the push to upstart biotechs that — whether out of financial necessity or inherent innovativeness — were looking for more efficient ways to bring new drugs to market.3
In 2002, the U.S. Food and Drug Administration (FDA) jumped on board when they announced a significant new initiative, Pharmaceutical Current Good Manufacturing Practices for the 21st Century. According to the agency, this document launched the FDA’s “vision of a maximally efficient, agile, flexible manufacturing sector that reliably produces high-quality drug products without extensive regulatory oversight.”
In his comprehensive overview of flex history,4 Jeffery Odum, vice president of Biopharma Life Sciences at Exyte, points to another significant milestone: In 2004, the International Society for Pharmaceutical Engineering (ISPE) published its Biotech Manufacturing Facilities Baseline Guide and in it, discussed an evolution in facility design. The guide demonstrated that moving from the more traditional open-system, classified space to a more closed-system design could provide enhanced flexibility in manufacturing options. Essentially, manufacturers could choose to contain their individual process steps that were vulnerable to contamination, rather than having to monitor the environment in the entire manufacturing space — this lead to cost savings and scheduling efficiencies.
From there, the concept continued to evolve as manufacturers pushed flexibility boundaries and equipment suppliers stepped up their games, introducing new enabling technologies, such as single-use systems. If processes were safely contained in closed systems, why not manufacture in a large space where you can wheel equipment in and out as needed? Why not run multiples processes in one space? This thinking gave birth to the ballroom concept — a large manufacturing area with no fixed equipment and minimal segregation thanks to the use of closed systems.
At the same time, monoclonal antibodies (mAbs) were becoming the bell of the biopharma ball as the modality’s vast therapeutic potential was contributing to its rising popularity. This worked out well, as large-scale mAbs were particularly suited for manufacture using this closed-system, ballroom facility design.
The most famed example of this paradigm in practice is Amgen’s Singapore facility, built with a focus on mAb processing. Formally opened in November 2014, the facility had all the flexibility bells and whistles. Using a modular and reconfigurable design, the facility was built in half the time of a conventional plant. Instead of custom equipment that’s welded together, the single-use equipment in the plant could be reconfigured, allowing operators to wheel in new equipment as needed. Through the use of technology and process efficiencies, the facility occupied about one-fifth the size of a traditional pharma plant but maintained a comparable level of output, and was able run at one-third of the conventional operating expense.
The plant, rightfully so, won multiple awards and was heralded as the “facility of the future.”
But the facility of the future is truly a moving target — and today, that target is starting to look different.
“The facility of the future — those large open ballrooms — were really intended to be for a monoclonal antibody process,” says JP Bornholdt, director of SlateXpace Technical Operations at CRB. “But cell and gene therapies have been a complete reawakening in what biotech really is. The industry has yet to determine a coherent vision about what the facility of the future looks like for advanced therapies and what those manufacturers will need — but flexibility is definitely part of that vision.”
As history has shown, even as pharma’s vision of the future shifts, the industry’s quest for flexibility is here to stay.
Flexing in pharma’s mirror
One important takeaway from the Amgen model is that in pharma, flexibility comes in many forms. Flexibility can be realized through aspects of facility design, technologies and equipment, and manufacturing processes.
According to Odum, this is one of the biggest stumbling blocks the industry has hit in its quest for flexibility. “One issue is the definition of flexibility. Is it driven by operational issues, products, other items? It is not always consistent between customers,” he says.
Determining what is driving the desire for flexibility is often the best place to start.
Some pharma manufacturers want a facility that can be physically scaled up or down to meet changing demands for products. Some want the ability to move equipment from one suite to another for quick changes in production plans. Some are striving for a multimodal facility that can be adapted to make different types of drugs.
Ken Anthony, who has 30 years of experience in engineering and construction for manufacturing, stresses the importance of having this discussion with clients. “You really have to dive into what the customer is ‘needing and expecting’ from flexibility,” says Anthony, who is currently vice president of Strategic Development at Binswanger, a commercial real estate and advisory firm.
The conversation is also happening internally for pharma companies.
“It ends up becoming somewhat of a philosophical discussion within the firm. It really depends on the output requirement and the goal of the individual facility,” says a director-level facilities manager at a major American pharmaceutical company. “For example, you can use a flexible facility to delay any larger capital spend for a product that may have a lower probability of success. Or, for something such as a gene therapy, flexibility of single-use equipment offers speed in terms of cleaning turnaround and validation.”
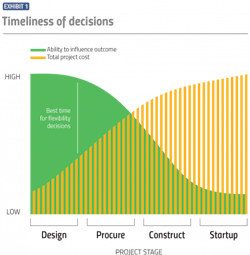
When it comes to costs, the timing of these flexibility-based decisions is important. As is the case with project design decisions in general, the earlier the better. The Construction Industry Institute uses a “cost-influence curve” to illustrate that it is much easier to influence a project’s outcome during the project planning stage when expenditures are relatively minimal than it is to affect the outcome during operation of the facility when expenditures are more significant. (See Exhibit 1).
Pharma companies and construction firms alike agree that developing a detailed plan of how flexibility is to be delivered upfront is crucial, but Anthony says it’s ideal to level-set expectations because even the best-laid plan isn’t foolproof.
“The concept of flexibility tends to morph over time. You may agree to what flexible means at the beginning of the project, but while you’re building the facility, circumstances change and all of a sudden your flexibility isn’t flexible enough,” Anthony says. “Because who knows what’s going to happen a year from now? How do you translate that uncertainty in the flexibility requirements for a facility? There’s really both an art and a science to it.”
The modular panacea?
A recent CNN article noted that one reason Pfizer was able to rapidly scale up COVID-19 vaccine production to millions of doses was the drugmaker’s strategy of using prefabricated, modular construction. Pfizer installed around 13,000 square feet of modular rooms in its Kalamazoo plant that had been pre-built in Texas and then shipped out to Michigan.
But the use of prefabricated modular construction is not a new strategy for Pfizer. In September 2013, Pfizer, along with partners GEA and G-CON Manufacturing, formally launched what the drugmaker called its PCMM concept — Portable, Continuous, Miniature, and Modular development and manufacturing — with the goal of adding more speed and flexibility through the use of prefabricated pharma manufacturing PODs.
The term “modular” — which can be used to describe both a facility’s design concept and equipment — commonly enters the flexibility discussion and at times, the two words are even used interchangeably.
Modularity can be achieved in a number of different ways. A modular build can be accomplished by assembling modular panels on-site; or the entire facility can be built module-by-module off-site, tested, taken apart, shipped and reassembled on-site; or, as in the case of Pfizer, facilities can be prefabricated — meaning assembled and tested off-site then shipped whole.
Bornholdt points out that an added bonus of some modular prefabricated units, such as modular cleanrooms, is that they can be considered capital equipment rather than capital improvements, and are thus depreciated at an accelerated rate, resulting in tax savings.
As the pharma industry begins to turn its attention more towards advanced therapy medicinal products (ATMPs), companies are managing highly complex product pipelines and are often unsure which modalities will move successfully through clinical testing, heightening the need for flexible solutions — and many companies are finding this through these modular builds.
“We found that a lot of our ATMPs clients had pipelines consisting of various combinations of gene therapies, cell therapies, autologous or allogeneic stem cells, etc. Especially when it came to contract manufacturers, they almost didn’t know what drug they’d be manufacturing next, or what their business needs were going to be,” says Bornholdt.
In these scenarios, the fast-paced construction project offered by modular preconstructed suites can allow capital expenditures to be pushed until later in the development cycle.
“If you committed to building a giant stainless steel facility five years ahead of time only to find out that a molecule in development failed, then you have a plant that’s at half capacity or less, and it’s either way undersized for the next drug or potentially way oversized,” says a senior engineer at a major American pharmaceutical company.
This delay in big capital spend minimizes investment risks, allowing companies to make more informed, better-timed decisions.
Yet, while modular is closely aligned with speed, it doesn’t always equate to flexibility. Once interconnected, modular facilities can become just as inflexible as traditional facilities. Additionally, Anthony points out that when it comes to prefabricated modules, the units are able to save construction time by using standardized designs. But when pharma companies require more customized designs, it complicates the situation.
“Modular is not inherently flexible — it was set up for rapid deployment and rapid scale up,” says Anthony. “Prefabricated modules are not really set up for you to start cutting into them and monkeying with them. If you go into a modular design and you start doing extensive customization, at some point, it’s no longer modular.”
Vendors of modular solutions are beginning to address this sticking point by finding the right balance between standardization and customization. The balance was not lost on CRB when the company introduced SlateXpace, a customizable turnkey facility solution based on the principles of flexibility and modularity. According to the company, the problem with offering a single standardized solution is that “when you try to fit everyone, you wind up fitting no one.”
“We use modular as a tool to gain advantages of flexibility and speed, but our SlateXpace approach is not limited to modular delivery. Over the course of nearly 350 advanced therapy projects, we’ve found the best practice is to start with a set of carefully planned design blocks based on function -— then arrange those design blocks to form a complete facility layout unique to each client’s process and manufacturing goals. That design is realized by blending the best attributes of various construction delivery methods — modular, panelized and stick-built — utilizing each in the appropriate location and ratio to deliver a facility that also meets the client’s particular business drivers, such as budget and schedule,” says Bornholdt.
Grabbing the flex fruit
It’s a classic case of “is the juice worth the squeeze” as pharma manufacturers evaluate the sliding scale between costs and benefits of different levels of flexibility.
“When it comes to building in flexibility, it’s certainly not impossible, but you have to keeping asking yourself ‘is it worth it?’ Flexibility comes at a cost which must be justified and included in the project budget,” says Brydges, who now serves as chief executive officer for the newly-launched company, Phylloceuticals.
Being a “pioneer” in flexibility isn’t necessary in all cases.
“Even within flexibility you need to take into account flexibility,” says a director-level facilities manager at major American pharmaceutical company. “In some cases, if you have a molecule that you know is going to be a blockbuster, that isn’t the way you need to produce it. Maybe you do need to look at economies of scale and ways to do that in fixed systems that actually make sense.”
But perhaps to the detriment of flexibility, new enabling technologies and award-winning facilities have painted a picture of higher first costs for flexible facilities. Yet, that is not always true and that line of thinking can hold the industry back.
“That is one of the problems. Flexible doesn’t need to mean ‘new’ anything,” says Odum. “Often flexibility can be achieved simply by optimizing current operational activities or by using simulation tools to better understand day-to-day needs.”
According to Bornholdt, manufacturers should first pay very close attention to overall cGMP facility flows (personnel, product, material, and waste movement) and how re-purposing or expanding will impact day-to-day operations. Moving towards closed processes and considering the appropriate level of segregation between unit operations are also important first steps, he says.
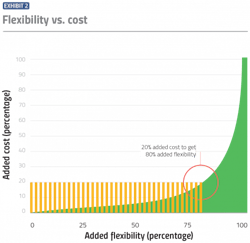
Anthony views it as an 80/20 rule.
“You can make most of the improvements needed to truly make your manufacturing plant adaptable and flexible, without having to spend a whole lot of money to do those things,” Anthony says. “I start with a goal of 20 percent additional cost to yield 80 percent greater flexibility.”
Most of the proverbial low-hanging flexibility fruit can be captured early on in the facility design project.
“There are things you can do technically to make a facility inherently more or less prone to change and problems,” Anthony says. For example:
Building knockout panels in walls
Knockout panels, which are a section of wall that can be removed (or reinstalled) without demolition, enable manufacturers to change plant capacity or move/add equipment as needed. This essentially embeds flexibility into the walls upfront, and means manufacturers won’t incur the costs of demolishing walls and moving existing equipment, pipes and electrical equipment.
“Knockout panels add no additional cost to the base building cost. On the flip side, if the owner decides later to move or add equipment and this was not planned for in the original design/construction, the costs can become exorbitant,” says Anthony.
Adding ample ceiling height and clearance
Rather than wrapping the facility tightly around equipment and interior spaces, manufactures can opt to add additional clear height — the distance from the finished floor to overhead objects — to a facility at the start of construction. This will help them avoid a situation down the line where new equipment or processes are needed and the building height cannot accommodate them.
“In this scenario, there is an added cost to the baseline building — maybe five percent more expensive. But the costs to ‘stretch’ a building after it is built are steep — and usually considered a constraint to proceeding. The opportunity cost in this scenario for the owner can be many times more than the whole cost of the original project,” says Anthony.
Bornholdt agrees and says in general, manufacturers should aim to design spaces that accommodate a wide range of equipment vendors and equipment scales. “Door and corridor clearances, ceiling heights, as well as power and utility requirements, should be confirmed against many potential equipment suppliers,” he says.
Plan for utility isolation or expansion
Installing appropriate and ample locations for utility isolation, shut-off and tie-in upfront can greatly reduce the operational downtime required for facility modification or expansion. “Utility, power and data use points can include blank ‘stub outs,’ ‘spares,’ and ‘rough-ins’ that may be filled in the future with little impact to ongoing operations,” says Bornholdt.
“There is an added cost here but it means you don’t have to shut down sections of the plant — or worse, the entire plant — when it comes time to add/change/provide maintenance on utility equipment,” says Anthony.
Additionally, other aspects such as foundation type and capability, floor coatings and HVAC management system configuration should be assessed for their level of flexibility.
Overall, many flexibility benefits can be captured from planning and forethought — without draining pharma’s wallets.
A flexvana future
As advances are made in manufacturing facility design, equipment, and processes, pharmaceutical companies are finding themselves facing an intimidating list of flexibility-related options. At the same time, the nature of the pharma industry itself is evolving, and new modalities continue to change the game for pharma manufacturers.
Fortunately, once the industry pushes past the buzzwords and gets down to logistics, the path to flexibility can be made smoother by establishing clear business drivers, making decisions early, and above all, recognizing that there is no magic flexibility pill.
References
1. Annette Kreuger 2021 (Jan 20, 2021). 2021 Industrial Market Outlook [Webinar]. Industrial Info.
2. F. Suarez, M. Cusumano and C. Fine. “An Empirical Study of Manufacturing Flexibility.” MIT Sloan Management Review. October 1995.
3. Hill, D., and M. Beatrice. “Biotechnology Facility Requirements, Part 1, Facility and Systems Design.” BioPharm International. 1989.
4. Odum, Jeffery. “Biotech Industry’s Quest for Optimized Manufacturing Facility Design.” Pharmaceutical Engineering. Jan/Feb 2020.
About the Author
Karen P. Langhauser
Chief Content Director, Pharma Manufacturing
Karen currently serves as Pharma Manufacturing's chief content director.
Now having dedicated her entire career to b2b journalism, Karen got her start writing for Food Manufacturing magazine. She made the decision to trade food for drugs in 2013, when she joined Putman Media as the digital content manager for Pharma Manufacturing, later taking the helm on the brand in 2016.
As an award-winning journalist with 20+ years experience writing in the manufacturing space, Karen passionately believes that b2b content does not have to suck. As the content director, her ongoing mission has been to keep Pharma Manufacturing's editorial look, tone and content fresh and accessible.
Karen graduated with honors from Bucknell University, where she majored in English and played Division 1 softball for the Bison. Happily living in NJ's famed Asbury Park, Karen is a retired Garden State Rollergirl, known to the roller derby community as the 'Predator-in-Chief.'