The country with the most FDA warning letters in 2019
Last year the FDA’s Center for Drug Evaluation and Research (CDER) issued dozens warning letters for manufacturing issues to pharma companies outside the U.S. One country in particular — India — received the highest amount of letters.
All told, CDER’s various divisions — including the Office of Prescription Drug Promotion Letters, the Office of Unapproved Drugs and Labeling Compliance, and others — doled out over 90 warning letters throughout 2019. But CDER’s office of Manufacturing Quality Letters issued 43 letters to companies outside of the U.S. Of those letters, 20 were aimed at facilities in India. China received the 2nd most manufacturing quality warning letters (11), while the rest of the letters were distributed among plants in Europe, Costa Rica, Singapore, Turkey and others.
Manufacturing quality issues in India and China have been well-documented for years. In fact, data from CDER shows that India has the poorest rate of FDA inspections with acceptable outcomes (83 percent) — much lower than China (90 percent) and the U.S. (93 percent).
Last year’s round of warning letters from CDER included a range of common violations including a lack of written procedures for processes inside the plant, various quality control issues, insufficient lab records, poor sanitation and data security. In one of the most egregious cases of the year, FDA officials found discarded CGMP documents and evidence of uncontrolled document shredding at Strides Pharma, located in Bangalore, India. Of all the companies listed for repeat violations, nearly all were in India or China.
Around a quarter of the overseas companies cited were involved in API production — one of the chief issues behind last year’s major recalls of several blood pressure medications such as valsartan. The companies cited also included some of India’s biggest manufacturers such as Torrent and Aurobindo Pharma.
The FDA has been facing increased scrutiny and pressure by lawmakers to alleviate the quality woes of foreign-produced drugs in the global supply chain. In October, CDER’s director, Janet Woodcock, was grilled at a Congressional hearing on what steps the agency is taking to improve quality issues related to India and China. As part of those efforts, the agency said it is working to increase foreign inspections and tackling its backlog of 965 foreign facilities that have never been inspected. By the end of 2019, the FDA said it had finally inspected about half of those facilities and removed 37 percent of the companies on that list because they had gone out of business or were no longer serving the U.S. market.
Source: FDA
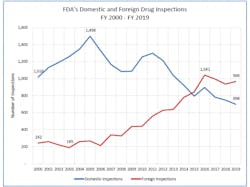
Since 2005, the FDA has been inspecting an increasingly higher number of global facilities — and according to an FDA report released in December 2019, the agency inspected more facilities overseas in FY 2019 (966) than in the U.S. (698).
In 2019, the agency employed 200 foreign inspectors, including 12 based overseas. The agency plans to add another 20 foreign inspectors to its roster and plans to fill all of the FDA’s current inspector vacancies. But, the FDA also pointed out that it can take between 1.5-2 years to properly onboard a new inspector.
The agency also hit dozens of U.S. companies with warning letters in 2019. Although CDER only cited one company in the U.S., the FDA’s various divisional offices and its four Division of Pharmaceutical Quality Operations offices issued over 100 warning letters to American facilities for various manufacturing, quality and misbranding violations.
America's fast-growing CBD industry was also hit with a number of warnings. Whole Leaf Organics, for example, was cited for selling unapproved new drugs, labeling violations, misbranding and making therapeutic claims that have not been approved by the FDA.