Turning A Buzzword Into Exponential Impact
Aircraft today can be fully developed in a digital environment. They are designed using CAD software and tested in a virtual flight simulator, before any physical work happens. Imagine the same in pharma: A COO can model various product portfolios, swap out machines, or model utilization and schedules to optimize agility and cost — all using software and delivering quantifiable answers in seconds.
Science fiction? Yes and no. The technology exists today — including predictive analytics, robotic process automation, and AI-based tools, all digitally connected via the Internet of Things (IoT) — but no pharma company has fully leveraged it. Some companies apply point solutions and individual tools, but most get stuck in the pilot phase and struggle to scale up digital across the enterprise. This approach leads to limited results that might excite the CIO but not the CEO.
Why is it so difficult to implement these tools at scale? You need visionaries — and not just pragmatists — to see the full potential of digital. At the same time, organizations need to manage expectations and understand the impact will come in successive horizons, not all at once in the next budget cycle. Finally, companies need to think of digital not as a series of individual tools but as a means of transformation, requiring technology and people.
The lessons of lean manufacturing
The lean methodology was invented by Toyota as part of the company’s famed Toyota Production System. Throughout the 1980s and 1990s, that system led to massive success in the automobile industry, with extremely high quality standards and lower costs, allowing Toyota to dramatically increase its global market share. In response, other car manufacturers — most notably U.S. companies like Ford and GM — tried to copy the principles of lean. Yet, their early efforts were high-profile disasters, in part because they tried to implement individual lean tools and processes without having the right organizational elements in place.
This approach was bound to fail. In fact, the actions of those companies were almost more disruptive than taking no action at all. One book, “The Machine that Changed the World,” recounts this struggle: “Lean-production methods on existing mass-production systems causes great pain and dislocation.” It took years for these manufacturers to realize that success in lean required more than merely plugging in some new technical tools or using fancy terminology. Rather, it required a fundamental rewiring of the organization and managerial system, which led to a cascading set of implications from the executive suite to the shop floor.
As with lean, the value from digital and AI will not come from trying to bolt tools onto existing processes. It will come through a comprehensive transformation of the entire organization. Currently, many pharma companies are at the earliest stages of experimentation, still limited to pilot tests, small-scale digital initiatives, and proof-of-concept exercises (or, as a recently McKinsey & Company paper published in conjunction with the World Economic Forum termed it, “pilot purgatory”). These are self-limiting; They will not generate the kind of dramatic results that leaders expect, potentially threatening future investments. If these companies are to take advantage of digital, they need to think in more comprehensive terms, by making systemic changes.
Why the future is different
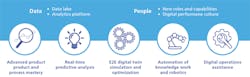
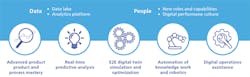
[pullquote]This kind of comprehensive approach is necessary because of five fundamental shifts in the way pharmaceuticals will be produced in the future. Collectively, the shifts are leading to a quantum change in manufacturing and order-of-magnitude improvements in processes.
1. Advanced product and process mastery.
First, companies can use new technologies to better understand how input parameters such as machine settings, operator training levels, or raw material options will affect quality and outcomes. In practical terms, companies can build an advanced analytical model and run historical data on chemistry, manufacturing, and controls (CMC) through the model to determine the impact of individual changes. By mapping outputs to inputs, companies can proactively optimize all inputs and thus reduce variations. In addition, by documenting this level of control over input parameters to regulators, companies can get rid of testing and thus cut throughput times in half. This obviously also boosts efficiencies, as most of the quality assurance and quality control tasks disappear.
2. Real-time predictive analytics.
Production managers start their day with a simple question: What is my biggest risk right now? Analytical models to predict critical events can answer this question. The risks can be deviations, quality issues, machine failures, or large changes in demand. Pharma operations executives can leverage big data, external and internal indicators, and machine learning algorithms to better forecast demand, and automatically identify and mitigate supply risks.
3. E2E digital twin simulation and optimization.
Companies can also build digital simulations of production processes — on the level of individual machines, labs, factories, or entire manufacturing networks (just like the aircraft example). These real-time digital twin simulations allow companies to steer processes proactively by predicting the effect of adding a new machine, changing schedules, or changing the team allocation — all before applying those steps to physical assets. In this way, the company can optimize production parameters for highly complex systems, accurately and proactively, without risk. This is a significant advance in efficiency compared to the traditional approach of sifting through historical data manually.
How is this different from existing planning and scheduling solutions? Real-time digital simulations offer dramatic improvements in speed and accuracy. “Demonstrated performance” for factors such as lead times or machine output replaces master data (which still today is of poor quality in most companies). Manufacturers can use real-time AI-based insights to evolve beyond simplistic rules like “frozen periods,” rhythm wheels, or minimum order quantities. Companies can run multiple simulations at the same time, allowing them to plan across multiple dimensions simultaneously, moving to multi-echelon planning. All this saves time and reduces the need for inventory buffers, so that companies can plan and sequence runs more effectively while staying focused on customer requirements.
4. Automation of knowledge work and robotics.
Finally, tools are emerging to automate and improve knowledge work and administrative processes. For example, digital robots can autonomously handle measures such as supply planning and scheduling, or use self-learning algorithms to support decisions such as portfolio margin optimization or corrective and preventive actions (CAPA). In the case of a product deviation, natural language processing can show where and what went wrong and compile this into a Pareto diagram or some other type of visualization. All these solutions reduce time — mainly of white-collar workers — and not by 10 percent, but by 90 to 100 percent.
5. Digital operations assistance.
The biggest weakness in pharma production is human error. Statistically, tasks performed by humans are about 92 percent accurate. This is incompatible with the compliance expectation in pharma. Therefore, the shop floor is increasingly digital, powered by new systems that support operators in daily tasks — particularly those that are highly repetitive. For example, tools such as augmented-reality glasses could show operators the checklist of steps needed to finish specific processes, or confirm that required measures have been completed, along with gathering and reporting data to fuel analytical models. Managers could also be given a tablet-based dashboard with real-time performance KPIs, losses, machine status, and potential measures to improve. If something goes wrong — or is likely to go wrong — the managers receive an alert.
The business case
Pharma supply chains have traditionally been characterized by long lead times (up to one year for the median pharma company) and high inventories (250 days). And although customer service levels are relatively high, most companies still spend a lot of time firefighting and balancing supply and demand issues.
So, what is the business case for digital? The technology is in the early stages, meaning any estimate is just that — an estimate. Yet, McKinsey research suggests that companies aggressively digitizing their supply chains can expect to boost annual EBIT growth by 3.2 percent and revenue growth by 2.3 percentage points. This impact comes from increasing both the efficiency and the effectiveness of supply chain planning and decision making. By connecting the supply chain end to end through real-time performance feedback and leveraging data from various sources (including ERP, MES, and external data), supply chain executives can generate greater visibility and improve performance.
Ultimately, digitizing the supply chain creates a strategic competitive advantage that boosts the supply chain organization from an execution-focused function to the center of business growth.
Three horizons to generate scale
Companies seeking to capitalize on these shifts typically start by launching small, targeted measures and then scale up across the organization. We see this process playing out in three horizons, with increasing gains in productivity and agility as the scope of projects grows.
In the initial phase — the experiencing horizon — the transformation is about launching use cases that are high impact but limited in scope, typically aimed at one specific unit or process. The objective is to build up experience and generate momentum. Even at this early stage, companies begin to see increases in operational efficiency, reliability, and agility. For example, based on recent digital plant transformation cases, we have seen:
- Reductions in deviations of up to 80%
- Increases in lab productivity by up to 60%
- Reductions in closures due to deviations of up to 80%
- Increases in OEE of more than 40% on packaging lines
- Reductions in changeover times by more than 30%
Once the company has gained some basic experience, it is time to shift to an exploring horizon. In this phase, companies launch “lighthouse” projects that demonstrate the full potential of a given technology and serve as an inspiration and innovation hub for the company as a whole. The scope covers the entire site, and the company starts using technology to differentiate itself against the competition. Typical achievements in the exploring horizon include prescriptive analytics (rather than descriptive), fully standardizing data across an entire production site, or a suite of advanced digital assistance to all operators at the site. It is critical in this phase to use models geared to value creation. Companies can expect an additional 50 percent increase in terms of efficiency, quality, and flexibility metrics.
Finally, companies can advance to an envisioning horizon, in which digital is rolled out across the entire value chain. By now, the organization has predictive machine learning models in place that can actively suggest optimization measures. Data is easily accessible and transparent, including secure e-validation from end to end. Many tasks have disappeared (such as testing) and regulatory and technology changes are simulated and approved. Advanced digital assistance tools are in place throughout all production sites (including those of suppliers and customers). At an envisioning horizon, companies can register a 100 percent increase across key operational metrics.
- Optimizing the supply chain through digital twins. Automatically updating master data and parameter settings with “demonstrated performance” and AI-based optimization tools leads to dramatic improvement in planning accuracy.
- Connecting the supply chain to increase visibility and improve decision making. Linking the entire supply chain end-to-end gives managers access to real-time performance information, leveraging data from various sources. That increased transparency boosts cross-functional collaboration.
- Better predicting demand and supply risks. Companies that leverage big data, external and internal indicators, and machine learning algorithms can more accurately forecast demand, as well as automatically scan and mitigate supply risks.
Key success factors
The reason many companies fall short in digital transformation is that they focus too much on the tools and applications, and not enough on building the right internal foundation. A truly transformative system change can only happen if companies have the conditions in place. To that end, there are several key success factors that management teams should focus on.
Invest in capabilities and adjust the organization
According to some estimates, up to 60 percent of manufacturing tasks can ultimately be automated by digital. However, that does not mean that 60 percent of jobs will go away. Rather, digitization will transform the way work gets done, scaling back the need for some positions but creating new demand for others. For example, data scientists, data engineers, and developers that specialize in user interfaces will be in high demand. Already, companies are fiercely competing for people with these skills, by launching dedicated internal capability-building programs, collaborating with universities, and revamping degree and certification programs. Moreover, companies are redrawing organizational lines to deploy digital production — for example, creating cross-functional teams that combine operations disciplines with IT. Others are realizing that the elimination of tasks in planning, analyzing, training, and supervision has implications on workloads as well as the required skills of their people, and they are rewriting job descriptions accordingly.
Apply strong change management
Launching a “digital transformation” can seem ambitious. But tentative half-measures will not capture the full potential from technology. Companies need to apply strong and deliberate change management through a comprehensive program that spans the entire value chain. Moreover, the transformation will disproportionately impact the middle layers and white collar workers in expert roles. Unless you have a vision and provide support to these colleagues — starting with a story that clearly communicates the need for change — the adoption of digital will stall.
Consider the perspective of a planner or process engineer at your company. After the first successful pilot of automated planning or AI used to predict batch outcomes, people in these roles will see that the processes they have spent years studying and improving can be executed by an algorithm in seconds, often with greater accuracy. It is inevitable that the sheer speed of information flow and transparency of performance data will create discomfort for many employees throughout the organization. Change management — starting with a change story, a strategic resource plan, and a reskilling program to build new cross-functional teams — can address this discomfort and ensure the overall program stays on track.
Rethink your approach to IT infrastructure development
Rather than thinking about IT projects as multi-million dollar, year-long transitions without a clear business case, companies should set up a common platform to connect all data sources (for example, through a data lake). The trick to connect more data in less time? Start with business needs. Once you have created the data lake as a foundation, you only create the 2 to 5 percent of connections that actually matter for the first generation of use cases. Transformations typically require 20 to 25 use cases before they generate sufficient momentum. Achieving this level of connection can take three to six months using a data lake — not three to four years as with traditional IT projects. This approach — also called “agile development” of IT infrastructure — is obviously a mindset shift and in some cases may not mesh with the existing IT roadmap that many companies already have in place. Unless there is clear agreement among the leadership team, this misalignment can quickly lead to delays.
Break the budget cycle mindset
Over the past decade, pharma operations have focused on reducing waste through measures like lean and sigma. Those efforts led to remarkable successes and sizable year-over-year cost savings — especially given the low cost of those measures. The downside is that some companies now have a short-term mindset. Digital holds tremendous potential, but it may not deliver gains on a predictable schedule that can be built into the budget cycle. Victories will require targeted investments in areas like IT, automation, and other technical solutions. Leaders need to help their teams distinguish between “budget tightening” initiatives and the investments needed to get to a digitally enabled future state. For this reason, some companies have created separate budgets managed by a leader with oversight over digital programs.
Digital manufacturing in pharma is not just a far-flung vision; increasingly, it is the industry’s new reality. Leading companies are developing a vision and roadmap for implementation, putting them on track to capture substantial value via gains in production capacity and reductions in unit costs. What is more, they are transforming their organizations to fully embrace innovation and position them to compete in a future that will look very different.
[javascriptSnippet]