“If you're proactive, you focus on preparing. If you're reactive, you end up focusing on repairing,” wrote John Maxwell, the #1 New York Times bestselling author and leadership coach. Maxwell’s comments are as relevant to a CEO as they are to a quality manager in life sciences — especially when it comes to making technology decisions.
Many life sciences organizations use legacy systems or paper-based processes for quality management. Difficult to manage, maintain, and scale, these manual solutions require a lot of overhead and limit visibility. Without the ability to easily share information, teams, sites, and departments across quality often work in siloes. As a result, processes take longer and it is challenging to be proactive or make informed, data-driven decisions.
Companies like Atrium Innovations are becoming more proactive by replacing paper-based quality management systems (QMS) with a modern solution to support worldwide sites and teams. This is especially important as globalization and outsourcing increase across all regulated industries.
For example, according to the FDA, 80 percent of U.S. drug ingredients come from abroad and nearly 40 percent of finished drugs are currently made overseas.1 Dietary supplement products and ingredients, a special category under the umbrella of foods, are also seeing rising imports. Approximately 80% of Vitamin C sold in the U.S. is now imported from China.2 More pressure is put on companies to comply with quality metrics regulations, as the FDA introduced the Quality Metrics Program for voluntary reporting on a specific set of quality analytics.3Realizing the potential challenges they faced, Atrium proactively chose a modern quality management solution better optimized for their business processes so they could get in front of these problems rather than react to them post mortem. Innovative technology “will have the potential to improve drug quality and safety,” said FDA Commissioner Scott Gottlieb, M.D.4 With modern solutions, companies can more easily collect, aggregate, and analyze quality data — enabling preemptive action and ending the reflex response cycle.
ATRIUM GETS PROACTIVE
Atrium broke the reactionary cycle and invested time to incorporate all functional areas and harmonize all sites. This initiative dramatically improved timelines for quality processes such as complaints, change management, audits, and product release.
Headquartered in Montreal, Canada, Atrium — a GMP manufacturer and distributor of nutritional health products — has operations in 50 countries and 1,400 employees worldwide. The company adopted a single, centralized, cloud-based QMS platform to bring together customer service, regulatory affairs, and quality assurance. Doing so enabled Atrium to manage complaints and manufacturing sites for collaboration and operational harmonization. Multiple, interconnected applications are now unified on one platform — a departure from sites working independently that may not be following best practices.
Access to information is key to managing daily challenges from change management to timely product releases to resolving complaints and audit-related issues. When teams are isolated in separate systems, doing so becomes more difficult. By providing real-time operational visibility and unifying cross-functional groups, companies like Atrium can break the reactionary cycle and improve complaint cycle times, timing of product releases, audit resolution, and change management processes in an increasingly global business landscape.
With limited information availability, many life sciences companies have difficulty tackling daily challenges. Change management decisions can be made with incomplete information. Managing complaints and driving them toward action or closure is onerous when teams are isolated in separate systems. Resolving audit-related issues can be difficult without transparency into audit results at sites around the world.
“We wanted complete transparency into each site’s operations and challenges,” said James Huang, director of global quality systems at Atrium. “If all sites can access the same information, teams can collaborate to expedite resolutions and save time. Adopting modern technology allows us to harmonize processes and bring together global parties for a more integrated and collaborative approach to making decisions in quality.”
RELEASING PRODUCTS FASTER
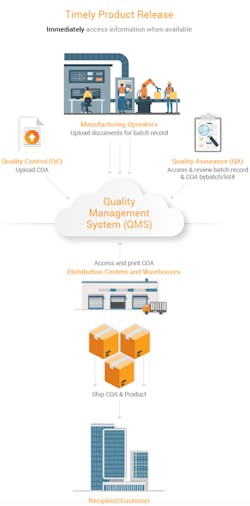
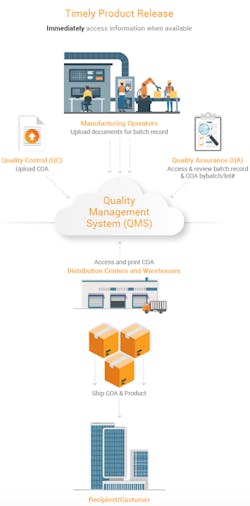
To release products, the quality assurance (QA) team needs to review batch records and supporting documentation such as a certificate of analysis (COA). Atrium improved management of batch records and COAs for timelier product releases.
Each department at the contract manufacturing site uploads batch record information and supporting documentation when it becomes available. The data is automatically filed to a centralized location, allowing stakeholders to see the status of each batch record even before it is finalized. QA can now easily review batch and non-batch related content, such as environmental monitoring, in one place. And for greater efficiency, teams can reference related items for all applicable batches. Once QA reviews the information and releases the product, all global sites, distribution centers, shipping and marketing operations, and regulatory affairs have immediate access to the COA from anywhere and can print the document.
This new reality for Atrium means that the organization saves time collecting and assembling batch record documents from different teams and eliminates the need to wait for a hard-copy COA that could be damaged or delayed, impacting the product release timing.
“The batch record is automatically assembled as documentation is available from the various teams. And, once the COA is ready, shipping can login from any location to print it. Real-time access to information allows us to make decisions and act faster,” said Huang.
DRIVING COMPLAINTS TO ACTION OR CLOSURE
In addition to making quicker decisions for product releases, Atrium wanted to seamlessly share information about product complaints from the customer service department with regulatory and quality teams. Ultimately, the quality team’s efforts resulted in success across the organization, enabling transparent, cross-functional decision-making for accelerated complaints to action or closure.
Atrium’s initial process required each department to re-enter or copy complaints from the customer service team into their own system. Duplication of complaint data was inefficient and slowed down the process. Realizing information delays could impact product disposition decisions, CAPAs, and timely patient safety reporting, Atrium’s quality team made complaint information more accessible for relevant stakeholders.
The team replaced a fragmented process with an integrated QMS, allowing Atrium’s customer service, quality, and regulatory affairs departments to share and access the same information about complaints in one place in the cloud. Organizations can identify complaint trends to be proactive and ensure CAPAs are created to take action or resolve issues.
“We’ve seen approximately a 50 percent reduction in cycle time from complaint to action or closure,” said Huang. “We have complete visibility into global and site-by-site operations. Since data is electronically collected, we can understand where delays occur and if they’ve been resolved. This gold mine of information means our quality teams can make better decisions with more complete data.”
Looking ahead, a unified quality management solution can also bring together medical inquiry, safety, and quality teams, further improving complaint handling.
GLOBAL REAL-TIME VISIBILITY INTO AUDITS
For efficient audit management, Atrium is managing and storing all audits in one place. Teams will have global transparency into audit findings for a site and can share critical findings or compliance risks with all other sites to prevent issue recurrence, mitigate risk, or collaborate on resolutions.
“Sharing critical findings with all sites allows us to quickly and collaboratively find the best approach to resolve issues,” said Huang.
With visibility into all sites for deviations, non-conformances, observations, and recommendations, including when they were recorded, updated, and resolved, organizations can more easily identify trends and proactively modify processes to improve operations.
Atrium has also been audited by health authorities and customers. With a single source of truth for all GxP content, the team delivered short turnaround times for any document request such as for a specific procedure, batch record, or specification.
“Auditors were very surprised by how quickly we could retrieve information. We can find any document in minutes,” said Dipti Dharia, vice president and chief quality and regulatory officer at Atrium.
MANAGING CHANGES EFFECTIVELY WITH TRANSPARENT INFORMATION
A recent PDA PAC iAM Task Force Survey5 revealed the challenges that companies face managing changes to products and their variations, as well as the impact that supply chain globalization has had on quality operations and collaboration. This research found that 65 percent of respondents manage 50 or more changes annually. For a given product, more than 50 percent of companies manage at least five product versions or variations, making the coordination of filings and approvals across all products and markets time-consuming. As the number of global parties has increased, it’s difficult for companies to quickly assess the impact of a change on their manufacturing, supply chain, regulatory, and other organizations.
A unified solution gives organizations like Atrium a structured process for receiving input from different geographic or cross-functional stakeholders—aggregating disparate change management data. All parties can have real-time transparency through the platform’s seamless orchestration and tracking of change management activities. For example, all functions are connected on one system so they can better understand the regulatory impact of a change, reducing the error-prone process of manual impact assessments.
This real-time visibility gives Atrium’s quality teams complete intelligence for making well-informed change management decisions. However, it’s not just about making good choices. Companies must manage hundreds to thousands of changes annually—how does a team prioritize which ones are most critical? The better the intelligence, the better companies can make the most productive changes, leaving the guesswork out of the equation. For Atrium, process improvement decreases risk and allows quality teams to be more agile.
Globalization has given quality teams further incentive to make decisions about changes with complete information. The PDA PAC iAM Task Force Surveyrevealed that when filing changes, more than half of the respondents indicated that they need to file in more than 25 countries. Also, an increasing number of foreign sites have been issued FDA observations. In the first two months of 2017, the FDA issued 11 Warning Letters to foreign drug manufacturing sites compared to 10 in six months in 2016, an almost seven-fold increase in just one year.6 Packaging, regulatory, quality, manufacturing, and supply chain teams worldwide can be more empowered in the face of FDA regulations if and when they have a complete understanding of the impact of a change on other sites and teams.
Working with other functional areas is also critical, allowing everyone to offer input, contribute data, and improve the entire operation. Almost 40% of the survey respondents said that at least half of post-approval changes require submission to a health authority, requiring collaboration between quality and regulatory. Atrium is looking ahead to implement change control across its quality and regulatory functions with a unified solution.
“A single cloud-based platform has transformed how Atrium works. We’ve propelled the company from working in siloes to a globally transparent environment where we collaborate seamlessly,” said Huang. “In particular, the idea of connecting and unifying not only our QMS and content processes but also our RIM management is very powerful. It will allow us to stay competitive in the market and continue to empower healthier lives.”
THINKING SMARTER, NOT HARDER: THE FUTURE OF QUALITY
The ultimate quality management principle still holds true: “Quality can be managed only if it can be controlled, quality can be controlled only if it can be measured, quality can be measured only if it can be defined.”
Atrium Innovations exemplifies how the future of quality management can define, measure, and control quality in real-time, enabling life sciences companies to make better decisions faster and more proactively. In the past 15 years, quality management teams have been caught in a cycle of reacting to unforeseen obstacles and repairing processes and technology — but the way forward will be different. Investing in modern, unified solutions will help companies gain the data they need for a holistic view of quality operations, understanding how events are tied to upstream or downstream processes.
By having all processes, content, and data on a single cloud platform, Atrium gained greater visibility into its manufacturing sites, and gave its geographically dispersed teams universal access to the same information. This real-time transparency accelerated their decision-making for change management, complaints cycles, product releases, and audit management. Atrium can focus less on logistics and more on meeting organizational goals and exceeding patient and industry expectations.
“With real-time capture of important quality information, we have greater transparency into sites and can aggregate data for global visibility. Using gathered data, we can track trends and gain better insight into the health of quality operations. It will also support proactive quality indicators, preventing small issues from becoming big problems,” said Huang.
Focusing on preparing and staying proactive is quickly becoming the benchmark for successful quality management. Companies will achieve lower overall quality management costs, fewer compliance problems, and significant time savings. They can also demonstrate to regulatory authorities that their operations consistently produce high-quality products — reducing the need for regulatory oversight.
SOURCES
1 “FDA Inspection Data on Nearly 1,000 Overseas Drug Firms is Missing,” by Rebecca Trager, Chemistry World, January 19, 2017. For more: https://www.chemistryworld.com/news/fda-inspection-data-on-nearly-1000-overseas-drug-firms-is-missing/2500277.article
2 “Eighty Percent of Vitamin C Is Imported from China. Is it Safe?” by Dara O’Rourke, Huffington Post, July 1, 2011. For more: https://www.huffingtonpost.com/dara-orourke/imports-from-china-safe_b_888977.html
3 “Submission of Quality Metrics Data; Draft Guidance for Industry; Availability; Request for Comments,” in a notice by the Food and Drug Administration, November 25, 2016. For more: https://www.federalregister.gov/documents/2016/11/25/2016-28332/submission-of-quality-metrics-data-draft-guidance-for-industry-availability-request-for-comments
4 “FDA in Brief: FDA Issues Guidance to Help Advance Novel Technology to Improve the Reliability and Safety and Help Lower the Cost of Pharmaceutical Manufacturing,” in a notice by the Food and Drug Administration, September 28, 2017. For more: https://www.fda.gov/NewsEvents/Newsroom/FDAInBrief/ucm577938.htm
5 “Post-approval Change and Knowledge Management – Where are We? Results from the PAC iAM Task Force Survey,” in a presentation deck by Emma Ramnarine at the 2017 PDA Annual Meeting, April 3-5, 2017. For more: https://www.pda.org/docs/default-source/website-document-library/workshops/2017/pac-iam/emma-ramnarine-pac-survey-results.pdf
6 “FDA Scrutinizing Foreign Drug Production Sites,” in a blog by Neil O’Flaherty, Baker McKenzie, March 8, 2017. For more: https://www.bakermckenzie.com/en/insight/publications/2017/03/the-usfda-is-scrutinizing-production-sites/