Designing the Next Generation of Quality Management Systems
As part of a maturing industry, pharmaceutical companies are under significant pressure to both innovate and successfully manage increasingly complex operations, more stringent regulatory requirements and frequent consolidations. Many are rethinking their quality management systems (QMS), recognizing the imperatives to enhance agility and improve responsiveness to market needs without increasing quality-related costs.
Creating a lean and agile QMS will be a key source of competitive advantage for these companies. Lean and agile systems have three characteristics:
- The ability to capture customer feedback and regulatory changes, build them into operations and launch new products rapidly.
- A streamlined structure that enables both compliance and operational efficiency, even when faced with increased business complexity.
- The flexibility to incorporate different modalities easily.
While the benefits are clear, a misalignment between the QMS and a company’s operational requirements can have downsides and drive costs. For example, a major U.S. automotive company with a strong quality reputation saw its JD Power IQS ranking fall by more than 15 places after introducing a new entertainment system in its vehicles. The system was complex to operate and frequently malfunctioned. The company responded with a multiyear effort that fundamentally changed how it thought about quality. In pharma, a similar misalignment can result in major quality or compliance issues that lead to hundreds of millions of dollars in remediation costs.
INDUSTRY TRENDS DEMAND A NEW APPROACH
Recent pharma industry trends have significant implications for QMS.
- Technology advances have increased the diversity of products and processes. Products may have more elements (for example, the drug itself, software and a device), while traditional product lines (small and large molecules) have matured and new processes are increasingly more complex.
- Mergers and acquisitions are bringing more and new modalities under the same corporate roof. It is fairly commonplace for companies to face the challenges of integrating QMS from multiple businesses.
- Regulators are using technology to gain access to data and tools that enable more frequent and more in-depth audits with an end-to-end scope. This increased scrutiny demands more extensive sharing of information and a greater emphasis on its integrity. At the same time, advanced analytics are greatly enhancing the ability of regulators and industry players to process this information and derive new insights.
Creating a lean and agile QMS to respond to those trends is not easy:
- Understanding end-to-end processes is challenging in a larger and geographically dispersed organization.
- Legacy QMS have become unnecessarily bulky over time and misaligned with business processes, due to incremental changes in response to quality incidents or audit observations. A pharma site has, on average, 100 to 500 change controls per year.
- Digital technologies and sophisticated data mining have changed the nature of products and innovation in the industry. Companies are increasingly moving toward providing end-to-end solutions comprising products and services.
- The widespread adoption of cloud-based solutions creates new challenges in redefining the paradigm applied to control changes and new-version releases without impeding innovation.
HOW TO DESIGN/IMPLEMENT A LEAN, AGILE QMS
Companies must take a set of steps to implement each element of a lean and agile QMS.
1. Capturing feedback and applying new insights in operations and development. Recent research shows that pharma companies lag behind other industries in capturing customer feedback and incorporating it into future designs. Many companies struggle with the implementation of regulators’ guidelines, resulting in multiple citations over the past few years.
Capturing voice of the customer. Customer feedback is no longer restricted to formal channels, such as product quality complaints and service reports. Capturing information from other avenues is increasingly a formal part of the QMS across industries, though pharma is lagging in this. For instance, consumer companies routinely track and respond to product issues on social media even before a formal complaint is raised. Digital tools can process large amounts of social media data to identify emerging quality issues early. These other avenues are also increasingly monitored by regulators and could trigger increased scrutiny.
Applying lessons across network operations. To avoid a repetition of issues, CAPA and governance systems are critical for enabling an organization to rapidly share lessons learned across the network. One company adopted a systematic process to evaluate the relevance of each audit observation to all sites, regardless of whether the observation involved central or site SOPs. The resulting “CAPA implementation matrix” not only reduced risk but also helped in harmonizing processes across sites.
Integrating and harmonizing QMS with development and operations. A company can facilitate speed to market by harmonizing the reviews (for example, through the use of similar metrics and formal forums in which R&D and operations participate) and using similar procedures during later stages of product development. For example, automotive manufacturers have developed shared modules and platforms across different car models to significantly accelerate the development process.
Leveraging the power of advanced analytics. Advanced analytics is enhancing companies’ ability to understand critical process parameters and material attributes during the commercial phase, resulting in demonstrated reductions in batch failures and deviations. It is equally important for companies to feed these insights back to the early development phase.
Establishing a high-performance quality culture. While the feedback loops are important to lay the foundation for agility, culture is critical for truly transformative change. Do employees handling customer complaints and feedback see their work as merely a compliance activity or as a critical input in the quality system? Are people rewarded for going the extra mile to collaborate across functions, including development and operations? Does the organization encourage a bias toward proactively addressing quality issues?
2. Streamlining the QMS
There are three types of complexity/inefficiency in QMS:
- Lack of coherence between the core business system and QMS processes
- Complex SOPs that are difficult to read and understand
- Extensive documentation requirements that limit productivity
The latter two issues are highly visible and apparent in day-to-day operations. Companies often respond by streamlining how SOPs are written and designing better documentation templates based on lean principles. But this approach does not radically change the QMS structure or thoroughly review its key elements. As a result, it is unlikely to be truly game changing.
In contrast, a lack of coherence between the core business system and QMS processes is more difficult to diagnose. The mismatch can be manifest in planning delays, slow decision making or low operational effectiveness. At one site, a culture assessment revealed that the mismatch between the QMS and business processes had reduced productivity, which in turn created pressure on the production system and increased quality risks.
Regardless of the product type or modality, many business processes are substantially the same. This makes it possible to design QMS with a relatively small number of procedures. For example, an Indian company’s sites had developed a large number of different quality control procedures that served the same purpose and could be reduced by 80 percent. McKinsey’s POBOS benchmarking suggests that a typical site has 40 to 60 percent more SOPs than its product complexity requires, and 30 to 40 percent more change controls per SOP than needed.
3) Making the QMS more flexible
We have observed four common QMS archetypes.
- One size fits all. Some companies use a single QMS that incorporates the highest level of regulatory standards to be followed by each modality. This archetype has the advantages of ensuring compliance by the entire organization, requiring only one quality organization, and driving the highest standards for each modality. However, it increases the regulatory burden and cost of quality and reduces flexibility and speed to market for some modalities.
- Modular. Other companies use a single QMS with different modules. Each product modality selects which modules are applicable. This archetype provides a fit-for-purpose QMS for each offering and can be managed by one quality organization. The ability to select only the applicable module enables agility and flexibility and increases speed to market. However, to avoid spending extensive time and resources to assess each product, the company needs a rigorous process to define the regulatory requirements for each offering up front.
- Hybrid. Some companies use a universally applicable QMS aligned to business processes at high level, but delegate responsibilities to teams and business units. This archetype ensures harmonization and enables the use of universally applicable SOPs, while also promoting flexibility by delegating responsibility to units that best understand product needs. This approach results in diminished oversight, because lower levels of the organization control compliance.
- Fully separate. In the fourth archetype, each modality has a fully separate QMS. This helps ensure compliance by each modality and promotes a leaner system within each unit is covered by a separate QMS. However, the use of this archetype results in a duplication of functions, increases administrative activity when transferring products across units, and reduces speed to market.
As this rundown of the archetypes’ pros and cons suggests, there is no “magic bullet” for addressing the challenge of designing a more agile, streamlined and flexible QMS. In any given situation, the right answer depends on the context in which the company operates and the maturity of the current system.
GETTING STARTED
A periodic reexamination of a QMS can identify significant opportunities for simplification. But it is important to avoid the trap of making incremental changes. Although a fundamental redesign of the QMS is challenging, it is essential in many cases.
To get started, a company should consider several questions:
- How are our operations changing? Which digital industrial technologies (known as “Industry 4.0”) is the company adopting and what are the implications for quality?
- What will our product portfolio of the future look like? Does the quality organization have the skills (such as relating to software or device quality) required to build quality into the products of the future?
- How is the QMS perceived? Do employees regard it as an enabler or as an unnecessary roadblock?
Finally, a company should recognize that expertise is not sufficient by itself to enable a successful QMS transformation. The company’s leadership needs to support and participate in the effort. The most successful and long-lasting changes are those driven from the top down, with every part of the organization truly called to action.
INTEGRATING QMS FROM MULTIPLE COMPANIES OR BUSINESS UNITS
In an industry where M&A has become commonplace, many pharma companies have faced the challenge of integrating QMS from multiple companies (which is similar to integrating separate QMS used by each business unit).
There are a wide range of integration approaches, including “full harmonization,” which requires significant time and resources and results in a complex system; “pick one,” which works well for similar product types; and “clean sheet,” which is used when none of the legacy systems is effective. However, based on our experiences supporting many companies in this process, we have identified five key lessons to take into account when integrating multiple QMS.
Lesson 1: A QMS is the way a company manages its business. Several sub-lessons flow from this:
- Quality objectives should be clearly linked to business objectives and strategy
- The QMS should be easily understandable, and should clearly establish the link between quality policy and ground-level execution
- Sufficient flexibility within the system is needed to incorporate business-unit or site-specific nuances and enable continuous improvement
- The system should enable the sharing of lessons learned and best practices across the business so that it can respond agilely to changes
- To readily adapt to regulatory changes, the system should be dynamic and have sufficient controls
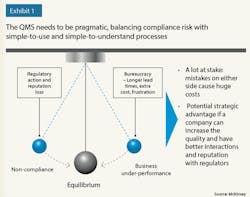
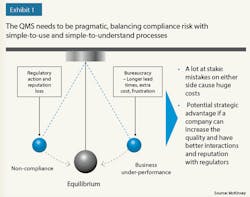
Lesson 2: A QMS needs to find the equilibrium between compliance and business goals (see Exhibit). Because there is a lot at stake, an imbalance can result in significant costs. To ensure equilibrium, a QMS should clearly articulate not only the quality compliance and business benefits, but also the cost of implementation and buy-in. Moreover, the QMS should be lean, both to enable adherence and avoid unnecessary costs and longer lead times.
Lesson 3: External best practice is the base case, and should be adapted to business and culture. Although the company should consider external examples, it must start by understanding its current performance and the adaptations needed.
Lesson 4: A QMS effort should have a risk-based prioritization of key factors that influence regulatory compliance. To avoid “boiling the ocean,” the company should design a very targeted and lean QMS, applying an external perspective on risk. It should also select the key processes to review first based on this risk assessment.
Lesson 5: A QMS should use a systems-based approach in which all the elements work well together. The company should take into account regulatory audit knowledge and expertise so that the QMS can be easily adapted without performing an audit of all the company processes. The company should also ensure that all elements of the QMS are well connected and work together effectively.