Dramatic changes in the pharmaceutical and biopharmaceutical landscape over the past decade have reshaped manufacturers' equipment and technology needs and purchasing patterns. The leading trends driving the transformation include the changing pharmaceutical pipeline, the shift to overseas manufacturing sites, new regulatory guidelines calling for use of the latest technologies to improve quality and efficiency, and a significant increase in outsourcing. Compounding these changes are continuous pressures to cut costs, improve efficiency, elevate quality and boost productivity. Together, these trends have had a significant impact on equipment and technology acquisition strategies.
STUDY RESULTS
According to the study, equipment quality and performance is the leading consideration of pharmaceutical manufacturers when making purchasing decisions, as companies seek products that will outperform others in quantity or efficiency. Interestingly, price ranks far lower in priority, following durability and reliability to ensure the equipment is dependable, and regulatory compliance at all stages of manufacturing with validation support that establishes evidence to demonstrate compliance. Customer service and products offering a comprehensive service agreement are of lesser importance.
MARKET TRENDS IMPACTING EQUIPMENT SOURCING
The changing nature of drug development, with an accelerating growth rate of specialty drugs, biopharmaceuticals and biosimilars and increased use of genetics, calls for new types of equipment and technologies. These sophisticated new therapies have impacted equipment purchasing considerations. Continuous production, advanced technology and flexibility through automation and single-use technologies are in high demand for manufacturing, processing and packaging. Companies also seek equipment that can manufacture and package these increasingly complex drug products with short start-up times and easy changeovers. They want highly efficient processing equipment that is easy to use from development to scale-up. Whether a company decides to make repairs to existing equipment or purchase new or used machinery, manufacturers need a reliable process that produces a quality product on a regular basis.
Generic producers and contract manufacturers require very robust, flexible machinery with high output, while complex medicines for targeted treatments demand flexible platforms and smaller batch sizes. Markets around the globe need safe, high-quality, consistent and highly efficient operations. Spending on equipment is focused on highly targeted technology and services.
Many drug manufacturers have shifted their focus to the development of new drug formulations and have outsourced their end production, such as commercial manufacturing and filling and closing operations, to contract manufacturers. The trend to more complex formulations has led to a higher demand for sophisticated technologies, while the trend toward small amounts of targeted drugs calls for flexible platforms that can handle small batches and ensure the highest safety for operators and products.2 With the rising pace of mergers and acquisitions, companies hope to reduce costs through synergies and access to new therapeutic classes or regional markets, as well as through consolidation of equipment and infrastructure. Interest in continuous processes is intensifying, and emphasis is placed on deploying equipment and technologies that enable higher production yields, a reduced need for purification and more rapid scale-up and commercialization.
Suppliers are responding with innovative technologies that meet these needs. At the same time, the surplus equipment market has experienced strong growth due to the increased availability of high-quality equipment and need for low-cost equipment solutions. Careful capital investment, cost-cutting, attention to operational excellence and efficiency, and optimal choice of an outsourcing partner are current strategies for success.Other industry trends in pharmaceutical processing and packaging equipment are the ability to handle potent substances, adapting lines for personalized medicine, the increasing use of disposable components, and anti-counterfeiting technologies. Single-use technology and continuous processing are the two key drivers in the bioprocessing industry today. Although each can be deployed on its own, the combination can bring even greater flexibility and gains in productivity.
COMMERCIAL SCALE SINGLE-USE TECHNOLOGY
Single-use, or disposable, technology is widely used in biopharmaceutical drug development and small- and mid-scale biopharmaceutical manufacturing, and more recently has been gaining acceptance in biologics production at increasingly larger scales. The growing interest in single-use technologies is driven by their advantages, such as lower capital expenditures and operating costs due to the reduction of cleaning and sterilization steps and the need for validation. Other advantages of single-use systems includes their greater flexibility, which allows companies to buy and assemble systems as needed and store them on site for future use. In addition, processes based on single-use equipment are more flexible, require shorter set-up times and have significantly reduced cross-contamination risk, all of which translates to a faster time to market and more robust, reliable production.Many types of single-use bioreactors are used to produce the major types of biopharmaceutical products, including recombinant proteins and monoclonal antibodies. Different designs are available for batch, fed-batch and perfusion reactions. While the initial focus was on developing disposable technology for upstream processes, single-use formats are now available for many downstream bioprocess steps, including filtration and chromatography. Disposable technologies are on the rise, offering optimal conditions for many manufacturing processes. However, the primary concern for the commercial market is safety, which must be the primary concern for biopharmaceutical manufacturers as single-use technologies advance to commercial, GMP manufacturing.
CONTINUOUS BIOPHARMACEUTICAL MANUFACTURING
Many newer single-use systems are designed for use in continuous bioprocesses, and disposable technology can enable fully integrated continuous biopharmaceutical production. Continuous manufacturing is attractive because it leads to more consistent products, processes and product quality; consumes fewer resources (raw materials, energy, water) and generates less waste; and reduces operating costs. Other advantages include streamlined processes, smaller equipment, low process cycle times, increased flexibility, more automation and less human interaction, and high volumetric productivity.3
To switch from batch systems to continuous processing, equipment and instrumentation must be modified and developed to handle a continuous flow of material from raw input to finished product.The switch may require new equipment, process control parameters, and control strategies to establish product equivalency.
For upstream biopharmaceutical manufacturing, perfusion -- where material is simultaneously charged and discharged from a processing system -- has become a well-established process that affords high quality biologic drug substances with high productivity and reduces product variability. BioPlan Associates predicts that in 2020, 50 percent or more of new bioprocessing lines and facilities will incorporate some elements of continuous processing, most likely in the area of perfusion.3 Other types of upstream equipment under development include continuous centrifuges, acoustic resonance devices and cell settlers.
For continuous downstream bioprocessing, simulated moving bed (SMB) chromatography and tangential flow filtration (TFF) systems are available and being adopted by the industry. New flow-through absorbers are also being developed for integration with chromatography and virus filtrations steps. Advances in process analytical technology (PAT) systems are also crucial to the successful implementation of integrated continuous bioprocesses. The industry has also recognized the value of flow-through chemistry for the production of active pharmaceutical ingredients (APIs) and continuous tableting for many years.
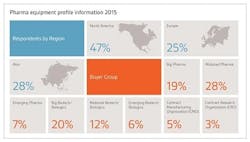
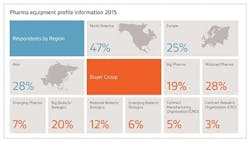
The Nice Insight Pharmaceutical Manufacturing Equipment survey was deployed to decision makers and influencers of biopharmaceutical equipment purchases within the biopharmaceutical industry. The 2015 report includes responses from 560 participants. The survey is comprised of 60+ questions and randomly presents ~30 questions to each respondent in order to collect baseline information with respect to customer awareness and customer perceptions of 98 of the top original equipment manufacturers (OEM), and providers of products, services and systems for the production of pharmaceuticals and biopharmaceuticals. Five levels of awareness, from "I've never heard of them" to "I've worked with them" factor into the overall customer awareness score. The customer perception score is based on six key influencers to purchase: Quality / Performance; Price; Durability / Reliability; Service Agreement; Customer Service; and Regulatory / Validation.
While the industry is increasingly producing highly sophisticated drugs and complex formulations, innovative, highly efficient new types of equipment and technologies are emerging to meet the changing needs. Capital equipment spending remains robust, focused on highly targeted technology and equipment purchasing. High quality performance and dependability are what most manufacturers seek when sourcing equipment.
To learn more about the report or about how to participate, please contact Guy Tiene by sending an email to [email protected].
REFERENCES
1. 2015 Nice Insight Pharmaceutical Equipment Report, Nice Insight, April 2015. More info at: http://www.niceinsightpharmaequipment.com
2. Rauschnabel J. Preparing for Pharma's Challenging Opportunities. August 6, 2014. Pharmaceutical Manufacturing. Accessible at:
http://www.pharmamanufacturing.com/articles/2014/pharmas-challenging-opportunities/
3. Hernandez R. Continuous Manufacturing: A Changing Processing Paradigm. BioPharm international; 28 (4). April 1, 2015.