It’s always exciting to see how a given industry responds to the market’s dynamics, and for pharmaceutical contract services companies these are exciting times indeed. For the longest time, this segment served Big Pharma from behind the scenes. Like Cinderellas, contract manufacturing, development and research companies (CMOs, CDMOs and CROs in the industry’s lexicon) were relegated to the back of the house, engaged at the servant’s entrance to serve in mundane and discrete roles, delivering API and excipients, clinical trial supply, or for some, extra commercial capacity to speed to market and meet the demand for a successful new therapy. For the most part, contract services companies became the towel boys and water carriers to the industry, certainly filling key and critical roles, but leaving the “competitive” lifting to their primarily vertically structured, predominantly capacity-heavy Pharma customers.
Globally the world Contract Pharma manufacturing market was worth an estimated $54.9 billion in 2013, up, says Visiongain, about $3 billion from 2012. Visiongain says its study covers a range of pharmaceutical contract manufacturing services from analytical studies to bulk API and FDF manufacturing. “Although some CMOs now offer development services, these have not been considered as part of the contract manufacturing market,” explains Visiongain, noting, “In recent years, growth in the pharmaceutical contract manufacturing market has been steady, despite increased interest in outsourcing throughout the pharmaceutical industry.”
What’s restraining growth? Visiongain points to three impediments:
1. Patent expiries for many blockbuster drugs, leading to lower demand for manufacturing.
2. Increased delays in clinical development programs, driven by a lack of funding for small companies and pipeline consolidation for multinational pharma companies.
3. Late-stage clinical trial failures for promising R&D candidates, leading to a lack of commercial supply agreements.
Visiongain breaks the pharmaceutical contract manufacturing market into three main sectors: active pharmaceutical ingredient (API) manufacturing, finished dosage form (FDF) manufacturing, and other services such as lyophilisation, spray drying and aseptic filling. In 2013, API manufacturing accounted for the greatest share of CMO revenues, with a market share of 68.1 percent. Using external suppliers for drug APIs has traditionally been more common than outsourcing FDF manufacturing, Visiongain notes. However, until the middle of the last decade, large pharmaceutical companies continued to produce more than three quarters of their APIs in-house.
RIGHT TIME, RIGHT PLACE
Sure the industry sustained itself along those lines for decades and it’s safe to assume that during the great AGE of the BLOCKBUSTER contract services providers, no doubt, fulfilled their respective roles with facility and economy, but mostly kept in their place, well within the bounds of their customers’ procurement operations.
Then THE WORLD ENDED for the pharmaceutical industry as we know it. Seriously though, the past decade has been an amazing one for Pharma. Who in the industry can’t recite the litany by now, describing the competitive landscape associated with end-of-the-blockbuster era and the emergence of new business models in response to enormous, practically tectonic political, competitive and regulatory pressures. During these turbulent times, the need to find cost savings, operational economies and competitive advantage prompted drug-owning companies to want more from its contractors, seeking deeper and more meaningful business relationships to help speed their products to market.
According to Visiongain’s “Pharma Leader Series: Leading Pharmaceutical Contract Manufacturing Organizations (CMOs) 2014-2024,” in the past 10 years demand for outsourced manufacturing services grew rapidly. Pharmaceutical companies, say Visiongain’s analysts, have sought to take advantage of the benefits of contract manufacturing — namely lower costs, increased flexibility and external expertise — while focusing resources on core competencies in drug development and marketing. “CMOs are increasingly seen as a strategic partner for pharmaceutical companies,” say Visiongain report authors, “providing a one-stop-shop of services for formulation development and manufacturing throughout the lifecycle of a drug.”
Roots Analysis offers similar insight from its study “Contract Manufacturing in the Pharmaceutical Industry, 2015-2025”: “Amongst the increasing financial pressures and the need to reduce cost and improve efficiencies,” says the executive summary, “the pharmaceutical industry has witnessed a paradigm shift from vertically integrated business model to a network of suppliers.” Contract manufacturing, note Roots Analysis’ report authors, explaining this segment of the industry has become an integral element of the pharmaceutical market. “Started initially as a one-off activity, [the contract services industry] has evolved into a dynamic business model; currently most prevalent in manufacturing, outsourcing is steadily spanning the entire pharmaceutical value chain.”
It’s well documented how established, branded Pharma acted (and is acting) in response to market realities, but there’s another part of this narrative that makes the industry’s recent history an even more compelling story: The rise of pharmaceutical contract services companies, “Contract Pharma” if you will, and their emerging operational leadership post blockbuster apocalypse as Pharma’s heavy lifters.
CMOs AND THE IMPERATIVES THAT DRIVE THEM
“There is a trend in the pharmaceutical industry of outsourcing activities such as drug and API manufacturing,” says a Visiongain analyst, remarking on drug owner outsourcing behavior in their CMO study. “Recent years have been lean for a number of the big players in the pharmaceutical contract manufacturing market, with M&A activity a core driver of revenue growth.” Each of the three contract services companies featured in the following pages, DPx Patheon, WellSpring and West are executing along those lines and doing quite well at it to prepare their companies to fight and win contracts.
Visiongain’s analysts illuminated recent CMO strategic positioning adding a note on the role operational acumen and technical excellence will play in delivering competitive agility: “The ability to deliver high-quality, flexible production will drive business to the leading firms in the market in the coming 10 years. Market-leading companies including Catalent and Lonza have placed considerable investment into high-potency API and antibody-drug conjugate facilities.” That last bit is very telling, pointing to where Contract Pharma is putting their money, not to mention that it’s in response to two HUGE drivers of contract services business, but more on that later.
Visiongain put it this way: “The market-leading CMOs have grown through acquisitions and site expansions to offer almost all required services on a global scale.” But Visiongain’s view digs even deeper, highlighting the role of Contract Pharma’s excellent niche players: “However, there is still a role to be played by specialist CMOs, particularly those that offer biological drug manufacturing services.” Visiongain says these areas are predicted to “experience long-term growth in demand over the next 10 years and will drive growth for firms with experience and production capacity.” Again noting that technical and operational excellence are muscles worth developing to achieve competitive strength: “Equally, areas such as lyophilisation and pre-filled syringes will see increasing demand from biopharmaceutical companies seeking CMO partners with specialist expertise.”
As mentioned, Contract Pharma’s future is being shaped by its customers who, says Price Waterhouse Cooper (PWC), continue to be attracted to outsourcing by several compelling reasons including: time-to market, cost advantages and risk management. Pharm Source principle Jim Miller, presenting at Interphex 2015, offered some insights into Contract Pharma’s market prospects and the potential opportunity it represents. Forecasting modest growth, Miller says the pace of growth has slowed down about a point, but that’s primarily due to the slightly slower pace of approvals, something that one might argue is expected in the wake of one FDA ’s best years as far as approvals go. Regardless, CMO revenues reached nearly $16 billion in the U.S. for 2014, according to Pharm Source. Regardless the future is brightening, and though Miller is relatively conservative, formulation complexity and the biologics/biopharmaceuticals sector are going to be presenting Contract Pharma with more than enough opportunities to flex their muscle.
CONTRACT PHARMA LEADERS
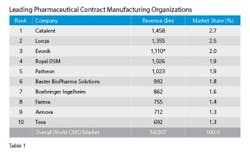
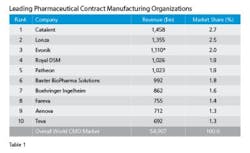
Visiongain’s analyst’s tallied revenues for the Top 10 leading CMOs pegging the group’s 2013 revenues at nearly $5.5 billion (See Table 1). Catalent, with its operational excellence and technical abilities well directed toward sterile injectables is number 1, enjoying 2.7 percent market share, which is a couple of points ahead of its nearest rival, Lonza. A quick scan of the Table, though, reveals that the spread among the Top Ten is not all that great, indicating a tight competitive market. Some say that is getting tighter because demand for capacity, needed within a tight or accelerated development cycle after a successful first trial, sometimes means business goes to the facility that is free to take it at the time — but only, mind you, if they have the technical chops and experience to successfully manage hard to manufacture solid dose formulations, the complex processing associated with biopharmaceuticals and emerging supply chain imperatives resulting from regulator’s serialization initiatives.
ORAL SOLID DOSE PROSPECTS
According to a recent study by That’s Nice, Nice Insight Research, a number of CMOs are prominent in Oral Solid Dose Forms (OSDs) including:
• AAI/CML
• Aenova
• Aesica
• Catalent
• Corden
• DPT/Confab
• Fareva
• Famar
• Patheon
• Pfizer CentreSource
• Recipharm
Of Nice Insight survey respondents, 25 percent indicated they will outsource commercial scale manufacturing projects. Identifying growth in this sector, 55 percent of this group will outsource solid dosage forms in 2014, up 4 percent from 2013. The survey also revealed that solid dose manufacturing will be outsourced at the greatest frequency (55 percent), followed by injectables (50 percent), semi-solids (44 percent), then specialty dosage forms (42 percent). Nice Insight found that in general, respondents reported they would outsource finished dosage forms (FDF) with greater frequency than API manufacturing. Regarding API work, 28 percent are planning to outsource small molecule API (28%) and 37 percent indicating large molecule projects in their future.
WHO'S OUTSOURCING SOLID DOSAGE FORMS?
Nice Insight found that 70 percent of Emerging Biotech respondents are planning to outsource solid dose formulations at (70 percent) followed by Big Pharma (60 percent) Mid Sized or Specialty Pharma (51 percent), Biotech/Biologics (50 percent) and Emerging Pharma (49 percent).
According to Nice Insight, 87 percent of solid dosage outsourcers will consider emerging market CMOs for the project with 63 percent already working with an emerging market supplier. The category is gaining traction. Nice Insight found 25 percent are aware of reliable suppliers in emerging markets but have not worked with one yet, and that 13 percent are willing to outsource to emerging market suppliers but do not know any reliable CMOs yet.
Nice Insight survey respondents say they are looking for assistance with a number of relevant technologies in support of their solid dose product development plans including Protection from Stomach Acidity (55 percent), Rapid Release (49 percent), Delayed Release (44 percent), Moisture Sensitivity (42 percent), Enteric or Targeted Release (33 percent), Multiple Colors or Branding (30 percent), Alternative/ Plant Based Polymers (24 percent) and Immediate Disintegration (20 percent).
What product types were of interest to respondents? Nice Insight says the FDFs are all on folks’ radar screens:
• Controlled Release Tablets – 62 percent
• Orally Disintegrating Tablets – 56 percent
• Immediate Release Filled Capsules – 55 percent
• Immediate Release Tablets – 54 percent
• Controlled Release Filled Capsules – 49 percent
• Powder Filled Capsules – 44 percent
• Resin or Bead Filled Capsules 33 percent
Finally, the likelihood of those planning outsourcing OSD development in the next year is strong among Nice Insight respondents who indicate that 15 percent are “Very Likely,” 47 percent are “Likely,” while 21 percent say they are “Undecided.” Only 8 percent responded “Unlikely,” with those indicating “Very Unlikely” at 9 percent.
LARGE MOLECULE DRIVERS
Eric Langer, principle at BioPlan Associates and author of the “11th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production” explains that CMOs are in contention, to a certain degree, for this segment of the industry outsourcing business — a notion supported by numerous indicators that biologics/biopharma is a growing market and offers rich ground for those CMOs capable of lifting those projects out of development and up to commercial scale.
Rottendorf Pharmaceuticals, currently producing 600 products for more than 200 clients, is a solid dose formulation specialist and a top 20 contract development and manufacturing organization (CDMO). Last quarter the company announced it won three formulation development projects and two commercial transfer projects with the Top Five pharmaceutical and biopharmaceutical companies. According to Rottendorf, the new projects reflect the industry’s positive response to its Total Process Ownership (TPO) business model, framing an emerging trend for CMOs looking to win an increasing share of the contract services market. Under its Total Process Ownership regime, Rottendorf assumes a higher degree of process responsibility and ownership — something that more and more drug owners are seeking due to reduced management oversight, reduced complexity and supply chain simplicity.
BioPlan’s most recent study, says Langer, reveals his respondents year-over-year increase in demand for Toxicity testing (87 percent, up from 75 percent), Fill/Finish operations (80 percent, up from 70 percent) and for Validation services (77 percent, up from 72 percent). Langer contends that these outsourcing activities have become mainstream and that his data suggests that the willingness to do so will only increase as time goes by. Langer says his study’s data also points to the fact that cost control is not as much as a motivating factor it once was. According to BioPlan, only 9 percent of respondents chose cost control as a justification to outsource manufacturing services, noting that the importance of CMOs demonstrating cost effectiveness has diminished year over year. Regardless, compared with other segments, BioPlan says outsourcing budgets are growing at an accelerating pace compared to other segments and that for the 11th Annual Report, respondents indicated budgets for outsourced biopharmaceutical manufacturing by 4 percent, something Langer notes is up significantly from each of the past five years.
IMPACT OF BIOSIMILARS
According to Langer, with more than 800 follow-on products in the pipeline, the interest and growth in biosimilars will provide a significant boost in business for those Contract Pharma players with the operational and processing muscle drug owners need. Langer explains that because many of these drugs have lower cost margins relative to biopharmaceutical drug innovators, he expects larger players to outsource their manufacture to CMOs.
According to BioPlan, there are currently some 40 recombinant proteins/antibodies with blockbuster (greater than $1 billion annual sales) markets and another 20 or so with sales from about a half billion on up. These are primary targets for development, says BioPlan, and that even just 10 percent of this market makes a very attractive market and thus a segment ripe for CMOs to exploit. He notes that new entrants into the market may also pursue an alternative business model, choosing to license-in follow-on products from smaller companies then turning around and outsourcing their manufacture. Langer says some of his respondents have shared that their biosimilars activity is up as high as 15 percent.
IT'S ABOUT RELATIONSHIPS
According to analysts examining the Contract Pharma industry, and Pharmaceutical Manufacturing’s own reporting, drug owning and drug innovating companies are increasingly seeing CMOs as strategic partners. Most of the recent studies have found that the quality of the relationship is a key indicator of how successful these alliances will be. Costs and price aside, both those buying and selling contract services know that the surest path to poor performance and increased risk is a poorly integrated, haphazardly planned relationship.