Sourcing Solutions for Environmental Monitoring: Ask the Right Questions
It simply makes sense for drug manufacturers to invest in technology to monitor labs, chambers, cleanrooms, warehouses, and other critical spaces in their facilities. But with no shortage of suppliers offering systems for tracking temperature, humidity and other key parameters, how do you know where to turn and who to trust? Which technologies and environmental monitoring systems (EMS) will meet your business and quality needs now and well into the future?
This article outlines an approach for sourcing an effective real-time environmental monitoring solution—one that will meet your requirements near- and long-term. By asking the right questions and collecting the right information, you can evaluate whether a solution will fit your purposes. Having a full scope of knowledge will save you time and, most importantly, bring high return from your investment.
Where to Start: Who Are Your Constituents?
Start with a systematic approach that targets your priorities for meeting business, quality, and regulatory requirements. Begin by seeking council from throughout your organization.
Chances are the solution will involve multiple responsibilities across your company, including quality assurance, facilities engineering, IT, operations, metrology, and purchasing. Including them in your project team from the start has several benefits. First, the decision to purchase a system will affect these various operational functions at some point, and early involvement can turn colleagues into supporters or at least non-dissenters. And it can help avoid problems down the road. Asking IT about network compatibility in the middle of your search, for example, can delay progress or even kill the project.
Second, anyone installing, using, maintaining or signing off on a capital investment will likely have set requirements or preferences that can influence the direction of a search. With each constituent, reach an understanding of basic requirements and current challenges. Document their expressions of wants and needs and how a solution could help get the job done more effectively.
Define the Project
Using data gathered from all parties, categorize requirements based on the risk to the business and product quality as specified by the Quality Management System. The result should contain a set of minimum requirements and a group of others relative to their importance to the company and various departments.
Once this is done, needs can be coalesced into a defined project. State the rationale for replacing or adding a new system and, where possible, refrain from specifying how to meet stated challenges. Systems will vary in their technology approaches and you want the flexibility to consider alternatives.
Develop a User Requirements Specification (URS)
While writing a User Requirement Specification is beyond the scope of this article, there are a few basic concepts to keep in mind. A URS should contain agreed-upon elements that will provide a consistent reference for vendors. It will document what you need the system to do. The project scope should have boundaries on the size of the facility, campus, or locations you want to monitor. The URS also needs to state the monitored environments such as warehouses, freezers, water quality systems and measured parameters such as temperature, humidity, pH, and conductivity. A supplier’s approach may vary on the number of sensors, types of parameters and their locations.
Clearly state your requirements. In fact, use the language of the FDA when something is required—it ”must” or “shall” have (fill in the blank). Try to leave little room for ambiguity. Later, the vendor can express ideas that you may not have considered, but the URS should at least bring readers to a similar interpretation of what you are asking.
What Questions Should You Ask?
The evaluation process often works in stages, especially when there is insufficient information to distribute a request for proposal (RFP). To assist this process you can send a request for information (RFI) to multiple suppliers. The questions below can serve as a template for an RFI, with the results feeding your RFP. Beyond the URS, your evaluation has to consider the strength of business partner supplying and supporting the environmental monitoring system. Ask open-ended questions to draw out how the supplier thinks and obtain ideas for addressing your challenges. Let’s review some questions.
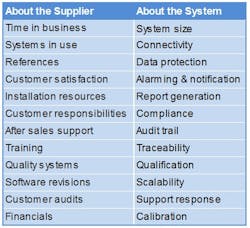
I. Questions about the Supplier’s Business
Equally critical to system capabilities is to understand the strength of a company in terms of their expertise, innovation and support. All three are important because you are likely making an investment decision that will span 10 years or more. A company with application or system experts that has little capability to innovate, for example, may leave you with a system that needs replacement before you expected.
Customer Depth
- How long has your company supplied environmental monitoring systems to GxP facilities? If it is a new entrant to the market, you want to know it. On the other hand, a lengthy history does not necessarily equate to quality, but these companies have learned a few things from their customers and have demonstrated staying power.
- How many of these systems have been installed to date and what percentage is GxP regulated installations?
- Who are three customers in related organizations that can be contacted for evaluation purposes? At a minimum, this lets you know that they have reference sites to visit and their proximity to you if you need to make the trip.
- How does your company rate itself in terms of customer support? This question tells you whether a supplier has a process for measuring customer satisfaction and incorporates feedback to improve its organization.
Business Depth
- What resources—dedicated, contracted, or both—are used to install, set up and validate the installation? It’s important to know the supplier’s capabilities to do the job and flexibility to meet your timeline.
- What maintenance capabilities does your company provide? You want to know the basics if they provide warranty, repair, and calibration. (See system questions for details about support and calibration.)
- What training is available for users and administrators and what documentation is provided? A system that is easy to use should have training programs that bring you up to speed quickly. In any case you need to satisfy personnel qualification requirements, CFR 211.25 for example, with documented training.
- What are the responsibilities of the customer to prepare for installation? The answer will provide insight into how organized the supplier is and whether it has a protocol for ensuring a smooth transition when the installer or trainer shows up at your facility. The list may also help you engage with other personnel in your company not part of the original project team.
Quality Systems
- What quality systems and requirements documentation does your company employ to ensure continuous improvement (e.g. ISO, certifications, accreditations)?
- What is considered a revision to the system and the typical release period for these revisions? This speaks to how progressive or systematic the company is about making improvements to the system. Too frequent updates may signal a problem in their understanding of market needs or can indicate quality problems. In validated applications, the operational benefits to upgrading have to outweigh the cost of revalidation.
- When was the last time your product development process or calibration lab was audited by a customer? How many times per year on average does this occur? Most audits are paper audits where email is the delivery vehicle. Though infrequent, physical audits by customers are good to know about, too.
II. Questions about the EMS
The requirements and capabilities of a solution can be understood by how a supplier describes what the system accomplishes for the customer. An answer that includes a value statement about the function can be equally important to a description of functionality.
Infrastructure
- What is the range of sensor points that the platform can handle and what are the performance tradeoffs with increasing size? This provides one indication that the system can conform to changing business needs. The system should scale from your initial project—50 sensors for example, to handle foreseeable growth (500, 5,000 or more sensors). Theoretically, a system may allow for unlimited expansion but, practically, there are limits. Increasing size may affect response time to alarms and data or require another platform.
- What connectivity options are available (wired and wireless 802.11 or 802.15)? You may have already specified a communications standard in your requirements. Depending on the type of facility, however, there may be occasion to mix hard wiring with wireless communications (WiFi or proprietary RF). Current or future operations may involve several plants with different infrastructures where it would be cost effective to use common hardware and software. You may be faced with budget issues of running wires and installing power where wireless is the preferred option. Conversely, if security or IT requirements limit connectivity to hard wiring, then you may have difficulties in supporting a wireless system.
- How are data protected during a power failure, network interruption or both? No system is failsafe but protection can be designed into a system to prevent loss of data or missed alerts to a temperature aberration, for example. Missing data is a quality issue whether there is a problem with environmentally sensitive products or not. Explaining to an FDA inspector why raw data is missing from a report leads to other questions that you may not be able to answer.
Alarming and Notification
- What is the flexibility for setting alarm limits, who can be notified, and by what means? You may have a protocol for alarm management or anticipate having to write or edit an existing SOP. Understanding alarm capabilities will help you decide. The system should provide functionality that best meets your need to record, notify, acknowledge and correct an alarm condition under different circumstances.
Consider scenarios. For example, rising temperature in a laboratory refrigerator sends email to a PC or activates a flashing light during a weekday and, at other times, a text message to security during off hours, a phone call to a manager on the weekend, and escalation when a response is not acknowledged. You want flexibility to meet personnel schedules with assignments to specific staff and physical areas, during one-time or recurring dates and time periods.
- What notification is available during a power outage, network interruption, or both? What happens when a freezer alarm triggers, for example? The answer should spell out options, if any, for local alarms or bypass to an independent external notification source.
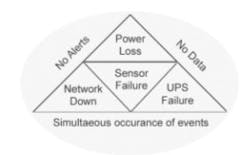
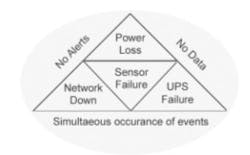
Fig 2. The perfect storm—concurrent failure of facilities and backup—can render even a “failsafe system” vulnerable to lost data or delayed alerts. Understanding these risks allows you to plan for contingencies.
Reporting
- How does the system generate reports, and who can create and receive them? This helps understand reporting and the flexibility to communicate data and results with others in the organization.
- What types of reports are available and how are they customized? Records should be available for different purposes and responsibilities. For example, a facility engineer may want a 6-month trend of data to develop a maintenance schedule for storage equipment. A quality manager may want raw data, summaries, and trends for a 24-hour time period to investigate a deviation. Reports should be easily accessible and easily understood without need for further analysis.
Computerized System Validation—Data Integrity
- How does the system ensure compliance with electronic records in terms of data alteration, unauthorized access and other data integrity measures? Documentation should be available to show the system is compliant ready with 21 CFR Part 11 or EU Annex 11. No supplier can claim in advance the system is compliant since this can only occur when it is installed in a validated environment.
- What audit trail information does the system provide, how can it be sorted, and can the audit trail be deactivated? The answer may be covered by the previous question on data integrity. Regulations require a review of audit trails based on risk. Depending on the level of risk, your quality system may require a review. Flexibility to sort the file by alarm, login, or other parameters can save time. Additionally, industry guidance recommends the system be incapable of disabling the audit trail.
- How is sensor (or instrument) calibration performed and what information is reported to prove traceability? Maintaining equipment with traceable records is required to ensure quality data and to be compliant. (See more on calibration below.)
- How is the system qualified? Assuming the system is validatable in that it is designed to meet compliance requirements for computerized systems, the supplier can indicate how this is accomplished. Once the system is shipped, you will need to decide who qualifies it for installation, operation and performance (IQ/OQ/PQ) in the application. Most customers do their own PQ but with resource issues you may want to know if the supplier offers this capability.
Cost of Ownership
- What is the typical time and resource requirement for adding a sensor or moving it to another location? Many systems provide the option of scaling up or down the number of points using in-house resources. This question can confirm what it takes.
- What support services are available including typical response time when there is a problem? Support services are like insurance. You don’t necessarily want to use them but want answers when there is a problem. Be realistic on your expectations. Response should always be prompt but highest when your facility or products are at risk.
- What mechanisms ensure that sensors or instruments maintain accuracy? Understand how instruments or sensors are calibrated. Investigate beyond the NIST certificate by understanding the supplier’s measurement practices. A system with stable and repeatable measurements between calibration periods can provide payback in time saved from frequent calibrations, field checks, or worse, sensor drift out of specification.
- What options are available for recalibration? Depending on your calibration practices, options can include on-site near sensors, in a calibration lab, performed by the customer or the supplier. Know your options and see if they work with your procedures or objectives for maintaining equipment.
There are other questions you can ask about the finer details of system operation. Comfort level increases with seeing the real thing even after you have received a sales demonstration. When you reach a point of due diligence that satisfies your criteria, ask the supplier to ship a demo system. And be sure you have staff committed to using it. There is nothing that takes the place of hands-on experience by future operators of the system. This exercise will generate additional questions.
Conclusion
The business benefits of sourcing the right environmental monitoring system for your organization can be enormous. Automating functions that previously relied on human intervention can lower risk and improve the effectiveness of personnel. Begin by ensuring that all relevant stakeholders are represented in your project team and get an understanding of their basic requirements. Asking the right questions is crucial. Accurate answers to the wrong questions pose risks and may unnecessarily drive up costs.
Armed with a consistent set of requirements, you can communicate sourcing criteria to the suppliers you want to evaluate. Use an evaluation method that embraces the whole solution. Combine system attributes with supplier capabilities to understand if a solution is fit for purpose and will continue to function with little disruption.
Evaluate whether the supplier can deliver accurate and responsive support when needed. While operation and maintenance should be easy, monitoring systems are complex and invariably need support. Is it easy to get support when you need it most?
Evaluate the strength of a supplier’s business: time in business, financial soundness, and what other customers say because the partnership should last a long time. More than once a monitoring system supplier has installed leading-edge technology only to depart the industry suddenly, leaving customers the job of premature replacement.
Gaining insight into how a supplier addresses your concerns, not just what they provide, will allow you to match an environmental monitoring solution with your business, quality, and compliance goals. You are in a risk-based business. Asking the right questions can mitigate risks in your controlled environments and reduce costs.General References
Institute of Electrical and Electronics Engineers (IEEE): www.ieee.org
Food and Drug Administration (FDA): www.fda.gov
European Commission Health and Consumers Directorate: www.ec.europa.eu
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH): www.ich.org
International Organization for Standardization (ISO): www.iso.org
About the Author
Ken Appel has provided marketing and market development in human safety regulated industries for over 15 years. He is a contributor to the bio-pharmaceutical industry on topics such as computer system validation, chamber qualification, warehouse mapping, environmental monitoring, and cold chain good distribution practices. Ken continues to enhance industry discussions on matters of regulatory and compliance through guest speaking at conferences, events and customer sites, as well as published works in the trade press. Mr. Appel is a member of the ISPE and PDA organizations. He holds a B.Sc. in Chemistry from the University of Massachusetts at Amherst.