Compliance and Traceability: Aligning Strategy and Infrastructure
Compliance and traceability go hand in hand. Organizations that emphasize compliance and have good track records in this area tend to be those that have established traceability in their manufacturing and regulatory operations. Those companies that emphasize traceability—in particular, that set up information systems and protocols that clearly document the history and performance of their operations—tend to be those that have stellar compliance records.
In November 2007, the Aberdeen Group surveyed and gathered responses from executives at 54 pharmaceutical companies about their efforts in compliance and traceability. A year later, we surveyed the same set of executives, with a slight increase of responses—up to 58 executives. (From the surveys we developed annual reports entitled, “Compliance and Traceability: Insight into Pharmaceutical Manufacturing.”)
We undertook these surveys in order to better understand the practices and technologies that are guiding compliance and traceability efforts within pharmaceutical manufacturing, in order to enhance our own knowledge of best practices for maintaining compliance excellence. These two sets of responses allow us to better understand drug manufacturers’ changing tendencies as well. One immediate conclusion can be drawn: While the pharmaceutical industry still lags behind other industries in terms of key metrics applied to this topic, it is catching up.
|
Traceability: One Man’s Definition “Traceability for our company starts with our supplier lot or batch of material and is carried through delivery to our customer. We have applied a QMS based on ISO-9001 with a twist to the pharmaceutical industry. This has GMPs built into the program and is supported by an ERP system. Shop floor data is presented real-time to the management team. A non-conformance control database is used to apply CAPA to process issues and customer complaints. The benefit for us has been effective claim resolution and continuous improvement support. |
Research from Aberdeen's 2007 report indicated that pharmaceutical manufacturers were behind the Industry Average in the adoption of quality management systems (QMS) and a number of additional Quality modules, and in a number of key performance indicators (KPIs). A year later, it is apparent that the pharmaceutical industry has made significant investments in infrastructure and is subsequently enjoying significantly improved performance.
In the following article, we will analyze the pressures driving these organizations to focus on compliance and traceability, the strategic actions they are taking in response, and the technologies being adopted in support of these strategic actions. We will examine how pharmaceutical manufacturers have closed the gap since last year by continuing to adopt technology in the support of compliance and traceability initiatives and aligning this technology adoption with the strategic actions being taken.
Compliance and the Production Process
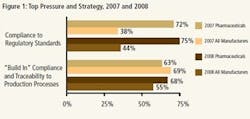
Results from both the 2007 and 2008 studies indicate that the tremendous pressure upon pharmaceutical executives regarding compliance has not changed (Figure 1). If anything, this concern has increased slightly in the past year (72% vs. 75%). When compared with counterparts from other industries, pharmaceutical manufacturing executives are almost twice as likely to face this pressure, a difference which affects many other aspects of pharmaceutical manufacturing operations.
It is interesting to note that the top strategy deployed by both the overall population of respondents and those in the pharmaceutical industry has remained the same over the past two years—to build compliance and traceability into manufacturing processes.
The one change that can be seen is that, in 2007, the overall market was more likely to be implementing this strategy, whereas at the end of 2008, the pharmaceutical industry is increasingly likely to be “building in” compliance and traceability to the production process. This no doubt can be partially attributed to the small up-tick in focus on regulatory compliance as the top pressure and the fact that "building in" compliance and traceability aligns well with the FDA's recent emphases on Process Analytical Technology and Quality by Design.
KPI Performance
In 2007, the only metric in which pharmaceutical manufacturers outperformed the Industry Average was in On-time Delivery (see Table 1 for metrics collected). Furthermore, in 2007, pharmaceutical manufacturers were only at par with the Industry Average for Compliance and significantly underperforming the Industry Average in regards to Response Time for Non-conforming Shipments.
Pharmaceutical manufacturers have shown significant improvement in 2008. This is very encouraging; in an industry in which customer safety is critical and the potential costs of non-conforming products high, any approach that underperforms the Industry Average is not sustainable.
When compared to 2007, pharmaceutical manufacturers in 2008 improved performance in every KPI that was measured, including: On-time and Complete Shipments, Products in Compliance, and Response Time to Non-conforming Shipments. In fact, pharmaceutical manufacturers now outperform the Industry Average in Products in compliance and overall equipment effectiveness (OEE).
Pharmaceutical manufacturers are clearly on the right performance improvement path but still have a ways to go before achieving Best-in-Class status.
Automating Compliance and Traceability
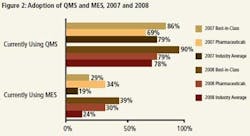
Changes in technology investments may help to explain where the current gains have come from and where future gains can be expected. Our report found a direct correlation between technology adoption and Best-in-Class performance. Best-in-Class manufacturers are considerably more likely to adopt manufacturing execution systems (MES) and QMS systems (Figure 2).In 2007, we saw that pharmaceutical manufacturers led the race when it came to MES adoption. However, even though these manufacturers were more likely than Best-in-Class manufacturers to adopt MES, the percentage, 34%, was still modest and did not increase year to year. In 2008, we now see more Best-in-Class manufacturers using MES. We believe that MES should still be considered a source of future competitive advantage, and those that have not yet adopted an MES system should do so.
Pharmaceutical manufacturers were less likely than both the Best-in-Class and Industry Average to have adopted a QMS in 2007, which could help to explain the industry’s underperformance in a number of KPIs. In 2008, we have seen a significant up-tick in the adoption of QMS among pharmaceutical manufacturers. It can be inferred that this increased adoption has helped drug manufacturers improve performance in metrics like OEE and Response Time to Non-conforming Shipments.
Technology Enablers
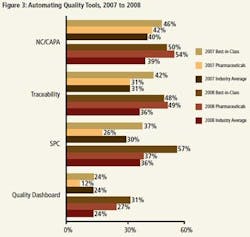
Beyond technology platforms, pharmaceutical manufacturers should look at specific enablers that are critical to realizing Best-in-Class performance. Many of the enablers shown in Figure 3 can be delivered by multiple technology platforms.All of the technology enablers highlighted in Figure 3 are more likely to be deployed by Best-in-Class organizations than Industry Average or Laggard organizations across both the 2007 and 2008 data sets. Because of this correlation to Best-in-Class performance, pharmaceutical manufacturers looking to improve performance and become Best-in-Class should pay them particular attention.
In additional to the broad technology categories highlighted in Figure 2, Aberdeen analysis found the top capabilities the Best-in-Class are adopting to address compliance and traceability are those highlighted in Figure 3. Adoption of quality dashboards enables manufacturers to display automated data collection across the value chain and use it as actionable intelligence, while adoption of methods such as non-conformance and corrective and preventive action (NC/CAPA), statistical process control (SPC), and traceability facilitates the real-time control of production processes and root cause analysis. These capabilities act as building blocks for an efficient compliance and traceability program. Manufacturers that have yet to adopt these modules should do so to help move up the competitive framework.
In 2007, pharmaceutical manufacturers were not competitive with the Best-in-Class and in some cases were below the Industry Average. We see improved adoption rates in 2008. Respondents indicate that pharmaceutical manufacturers are now ahead of the Industry Average, and in some cases the Best-in-Class, in the adoption of these technology enablers, all of which correlate to achieving Best-in-Class performance.
Eye on Improvement
The following recommendations will enable pharmaceutical manufacturers to improve operational performance and ensure future success for their compliance and traceability initiatives, ultimately allowing these manufacturers to achieve 100% compliance with government regulations while still achieving Best-in-Class operational performance.
- Focus on "building in" compliance and traceability to production processes. Deploying this strategy leads pharmaceutical manufacturers to adopt technology and focus on KPIs that help achieve Best-in-Class performance. It also helps satisfy FDA requirements.
- Adopt MES and QMS solutions that provide core track and trace capabilities. Aberdeen has found that manufacturers adopting MES and QMS are more likely to perform at Best-in-Class levels. Pharmaceutical manufacturers that have still not adopted these solutions should begin to do so as soon as possible.
- Drill down to improve operations with automated NC/CAPA, SPC, and Quality Dashboards. These technology enablers help organizations gain real-time control of production processes, have more successful root cause analysis, and ultimately improve decision-making.
Compliance and traceability are about more than just filling out checklists and tracking materials and products across suppliers and customers. It is about closing the loop when a non-conforming event occurs with root cause analysis. It is about controlling production processes in real-time to predictable tolerances. And it is about providing visibility to all of this at the right time to the right decision maker, all of which is made possible through automated NC/CAPA, SPC, and Quality Dashboards.
About the Author
Matthew Littlefield is a Senior Research Analyst for the Manufacturing Practice at Aberdeen Group. In this position he studies how manufacturing enterprises manage processes, people, and technologies to address continually changing market pressures. Littlefield's research identifies the best practices of the most successful firms, which is then presented within the structure of Aberdeen’s competitive market framework. His areas of expertise include: Manufacturing Operations Management, Enterprise Manufacturing Intelligence, Enterprise Quality Management, Lean, and technology interoperability.