Evaluating the pharmacological, pharmacokinetical and toxicological properties of a drug candidate during early stages of drug development requires developments of liquid formulations. In order to solubilize compounds that are poorly soluble, solubilizing excipients need to be added. This needs to be done without compromising the concentration of the active pharmaceutical ingredient (API) required to treat a condition at a therapeutic level.1,2
Traditionally, selecting excipients for their solubilization properties has been a manual process, relying on a trial-and-error-based approach to find the most suitable candidate. In the interest of saving time and reducing costs, drug developers are now looking for alternative ways to identify appropriate excipients. What follows is a detailed discussion of how a new high-throughput screening (HTS) method could help reduce the time and eventually the cost taken to find excipients that solubilize and stabilize compounds in liquid form.
EXCIPIENT SELECTION: CURRENT APPROACHES
Solubility is a critical component of drug development as the drug must be soluble to achieve the therapeutic effect. There are many methods used to enhance drug solubility, most of which adopt a trial-and-error-based approach, which is both costly and inefficient. Added to this, the frequency of encountering compounds with low solubility properties is likely to increase as larger and more complex molecules enter the drug development pipeline. As a result, there is a clear requirement for a more intelligence-based methodology to help overcome this challenge.
Although these methods are often successful, they are manual processes which tend to take between three and four weeks and demand large amounts of expensive API material often not available at drug discovery and clinical trial stages. Not ideal for drug development companies looking to get to trials with a robust formulation.
DEVELOPING A HIGH-THROUGHPUT METHOD
Given the nature of screening for multiple excipients solubilizing capacity, it’s easy to see why automating the process could be beneficial. Speeding up excipient selection could cut costs and timelines, as well as potentially reducing the number of tests required to find a suitable candidate, meaning less API is needed at this phase of the development process.
With this challenge in mind, a new HTS method designed to provide a more efficient way of screening an excipient’s solubilization capacity has been developed. The platform has the potential to help drug developers overcome the challenges associated with traditional techniques and provide conclusive information surrounding the chemical stability of a compound in different solvents and excipients.
Prior to establishing a platform methodology, several experiments were performed to optimize the approach. A screening list of excipients with a diverse range of solubilization properties, including water-soluble organic solvents, non-ionic surfactants, water-insoluble lipids, organic liquids/semi solids, cyclodextrins and phospholipids was developed6 to incorporate as many variations as possible.
The approach involved testing the interactions of 30 different excipients with six commercially available drugs, all with a diverse range of properties. These experiments ran in parallel in 96 well-plates, with each formulation being dispensed by a Tecan automated dispenser. The plates were then shaken for 48 hours to ensure equilibrium. The solubility results from the HTS were then compared with those achieved using a manual shake flask.
The method is based on identifying the solubilization capacity of each excipient for a specific compound and selecting the optimum formulation based on the results.
EXPERIMENTAL FINDINGS
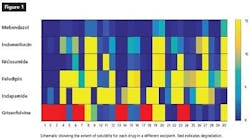
The results of the project can be seen in Figure 1. While some of the excipients demonstrated better solubilization capacity than others, the results indicated that the automated HTS approach identified the ideal excipient/s for each drug as effectively as the manual method. In addition, the approach was shown to be successful in providing a range of solubility information as it was tested using the manual method. However, the amount of work and time consumed is far more using the manual method than the HTS method.
Encouragingly for drug developers and indeed the industry as a whole, this innovative high throughput approach also generated data much faster than traditional methodologies, offering the potential to cut testing time from three to four weeks to between three and five days. Naturally, this kind of breakthrough offers huge benefits to drug sponsors in terms of shortened timelines and quicker data generation, which will ultimately impact a company’s clinical pathway.
ESTABLISHING NEW STANDARD METHODS
A drug compound’s solubility determines whether it can be turned into an effective drug candidate and as such is a vital component of drug development. Ultimately, automating the process of selecting a solubilizing excipient for a compound can save time and cut costs while allowing drug developers to take new therapeutics to market more efficiently.
The HTS method developed as a result of this project, currently applied at Recipharm as the first stage for liquid formulation development, is capable of identifying the best excipients to solubilize a compound in order to test its toxicology profile, bioavailability and pharmacokinetics characteristics. Also, chemical stability of the compound in different media can be informed by the HTS method.
By offering faster turnaround times and more detailed information on a compound, while also using significantly less materials than the manual alternatives, drug companies are presented with a unique opportunity to improve their formulation strategies and speed up the drug development process. We believe that this is the technology of choice after discovering a new compound and that it will become a standard method in drug development.
REFERENCES
1. Li, P., & Zhao, L. (2007). Developing early formulations: practice and perspective. International Journal of Pharmaceutics, 341(1), 1-19.
2. Dahan, A., Beig, A., Lindley, D., & Miller, J. M. (2016). The solubility–permeability interplay and oral drug formulation design: Two heads are better than one. Advanced drug delivery reviews, 101, 99-107.
3. Jornada DH et al. (2015 Dec) The Prodrug Approach: A Successful Tool for Improving Drug Solubility, Molecules. 29;21(1):42. doi: 10.3390/molecules21010042.
4. A. V. Yadav (2009 Jul-Aug), Co-Crystals: A Novel Approach to Modify Physicochemical Properties of Active Pharmaceutical Ingredients, Indian J Pharm Sci. 71(4): 359–370. doi: 10.4103/0250-474X.57283
5. Drug Discovery Today, Volume 12, Issues 23–24, December 2007, Pages 1068-1075, Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs
6. Strickley, R. G. (2004). Solubilizing excipients in oral and injectable formulations. Pharmaceutical research, 21(2), 201-230