Oral solid dose formulations are the pharma industry’s tried-and-true treatment forms.
Tablets and capsules are efficient and cost-effective to manufacture, shelf stable and easy to administer. They are widely understood and embraced by patients worldwide.
Despite billion-dollar large molecule injectable drugs like Humira and Keytruda topping the market charts and a pipeline buzzing with excitement over biologic hopefuls using novel scientific approaches such as CAR-T cell therapy, small molecule solid dose drugs still make up the vast majority of drugs on the U.S. market.
And, for the past two years, new drug approval for tablets and capsules specifically have outstripped approvals for injectables, solutions and creams.
Adding to this, several small molecule OSD products are beginning to show promise in the fight against COVID-19, thrusting aging medications back into the spotlight and renewing patients’ and manufacturers’ enthusiasm for OSD drugs.
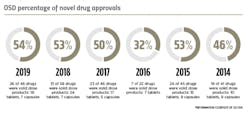
New drug approvals
When it comes to novel drug approvals, oral solid dose products continue to lead the charts. In 2019, the U.S. Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) approved 48 novel drugs. OSD products accounted 54 percent of these new FDA-approved drugs. Of the 26 newly approved OSD products, 19 are tablets and seven are capsules.
This is the highest percentage of OSD approvals seen since 2013.CDER identified 20 of the 48 novel drugs approved in 2019 as “first-in-class” — drugs that use a new and unique mechanism of action for treating medical conditions. Ten of these innovative drugs were OSD products. One notable approval was Janssen Pharmaceutical’s Balversa (erdafitinib) tablets, approved under the FDA’s accelerated approval program in April 2019 as a treatment for adult patients with locally advanced or metastatic bladder cancer. The first FGFR kinase inhibitor to receive FDA approval, Balversa is an important new therapy for a small subset of patients with urothelial carcinoma who, up until this approval, had limited treatment options.
Novartis topped the oral solid dose approval chart in 2019 with three novel drug approvals. These approvals included: Egaten tablets (Feb. 2019) to treat Fascioliasis, a neglected tropical disease; Mayzent tablets (March 2019) to treat adults with relapsing forms of multiple sclerosis; and Piqray tablets (May 2019), a targeted treatment for advanced breast cancer.
The FDA also approved a total of 1,171 generic drugs in 2019 — an all-time record. Importantly, 107 of these approvals were “first generics” — meaning the manufacturers were the first to get approval to launch generic equivalents. Close to 60 percent of these first generics were OSD products.
Market moves
GlaxoSmithKline kicked off 2019 by completing a $5.1 billion acquisition of Tesaro, an oncology-focused company based in Waltham, Massachusetts. Driving the deal was Tesaro’s biggest product, Zejula capsules, oral poly ADP ribose polymerase inhibitors approved for use in ovarian cancer.
Last summer, Novo Nordisk bought a state-of-the-art manufacturing plant for solid oral dose product from Purdue Pharma. The North Carolina facility enabled Novo to establish tablet production capacity in the U.S. in order to build a domestic supply chain for its oral semaglutide, the first glucagon-like peptide-1 (GLP-1) receptor agonist available in a pill form.
Around the time same, Amgen paid Celgene $13.4 billion for the rights to Otezla (apremilast) tablets. The tablets are the only oral, non-biologic treatment for psoriasis and psoriatic arthritis — ailments normally treated via injection or creams.
In recent news, India’s Piramal Pharma Solutions announced that the CDMO will acquire a solid oral dosage drug product manufacturing facility located in Sellersville, Penn. from G&W Laboratories. This acquisition is important because it gives Piramal solid oral dosage form capabilities in North America.
Recalls
Oral solid dose products had their share of struggles in 2019 as well. A series of recalls from 2018 spilled over into 2019 after regulators found several different toxic chemicals in the commonly used “sartan” blood pressure tablets — valsartan, losartan and irbesartan. Multiple drugmakers initiated recalls of tablets found to be contaminated with NDMA, NMBA or NDEA — all potentially cancer-causing chemicals.
This was followed by a mass recall of Zantac and generic Zantac (ranitidine) capsules and tablets because of confirmed contamination with NDMA above levels established by the FDA.
The bulk of the contamination in both scenarios was traced back to active pharmaceutical ingredients (APIs) being sourced from specific factories in China. In the case of Zantac, testing determined that levels of NDMA increased over time depending on how the ranitidine was stored.
The contamination was part of what has emerged as a much larger issue — the U.S.’s dependence on pharma ingredients made in China — that has gotten heightened attention during the coronavirus pandemic.
Fighting COVID-19
The pandemic drove non-COVID patients, fearing possible infection, out of clinical settings, which made administering drugs with complicated delivery methods difficult, if not impossible — again highlighting an important advantage of easy-to-use OSD products.
Adding to that, while most of the world’s attention is focused on developing possible SARS-CoV-2 vaccines, numerous OSD products have begun to share the spotlight as promising treatments for the symptoms of COVID-19.
Small molecule oral solid dose products, such as hydroxychloroquine (used in malaria and lupus treatment), chloroquine (used in malaria treatment), azithromycin (informally known as a Z-pak), lopinavir/ritonavir combos (used in HIV treatment) and colchicine (used in gout treatment) continue to be tested as possible COVID-19 treatments.
Given the scope of COVID-19 infections, ease of manufacturing, distribution and administration of tablets and capsules add to their attractiveness as potential treatments. But because some of these medications are decades old, and have previously been used for rare indications, most have only been produced in smaller batches — so if proven to be effective against COVID-19, manufacturers will need to reconfigure their production for large-scale output.
According to a blog post by solid dose expert, Dave DiProspero, director of Pharmaceutical Process Technology at CRB, this potential surge in demand “could hail a long-term shift for OSD manufacturers.”
“By taking decisive action to upgrade and expand their operations, OSD manufacturers will position themselves to play a key role in defeating both today’s pandemic and whatever unseen health threats the future may hold,” said DiProspero.
Oral solid dose products continue to play a pivotal role in the industry’s quest to produce quality, effective treatments for myriad diseases. And, now at the mid-point of 2020, just over half of the novel drug approvals are OSD products — signaling there is even more innovation to come.
About the Author
Karen P. Langhauser
Chief Content Director, Pharma Manufacturing
Karen currently serves as Pharma Manufacturing's chief content director.
Now having dedicated her entire career to b2b journalism, Karen got her start writing for Food Manufacturing magazine. She made the decision to trade food for drugs in 2013, when she joined Putman Media as the digital content manager for Pharma Manufacturing, later taking the helm on the brand in 2016.
As an award-winning journalist with 20+ years experience writing in the manufacturing space, Karen passionately believes that b2b content does not have to suck. As the content director, her ongoing mission has been to keep Pharma Manufacturing's editorial look, tone and content fresh and accessible.
Karen graduated with honors from Bucknell University, where she majored in English and played Division 1 softball for the Bison. Happily living in NJ's famed Asbury Park, Karen is a retired Garden State Rollergirl, known to the roller derby community as the 'Predator-in-Chief.'