Biomagnetic Separation: Thinking Bigger, Part II
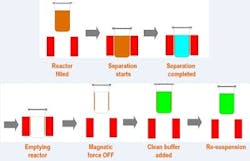
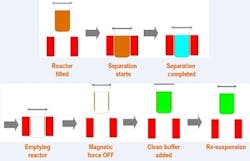
Biomagnetic separation has many advantages as a biopharmaceutical downstream purification technology. When using magnetic beads coated with the right biomolecules, the protein of interest can be captured within seconds and retained by a magnetic force while the supernatant is washed out. Once the magnetic field is removed, the beads can be re-suspended in a clean buffer. The protein can then be eluted and the magnetic force applied again. The beads will be retained and the extracted supernatant will only contain the purified protein in the clean buffer. By removing the magnetic force, the separated magnetic carriers can be re-suspended in the appropriate buffer and reused several times.
The usual reaction is to blame the magnetic beads, which initiates a series of long (and unpleasant) discussions with the providers. Nevertheless, new magnetic beads, buffer changes or applying longer separation time are useless. The process never reaches the efficiency necessary for a real-world application. These frustrating experiences have spread the idea that magnetic separation is not a suitable technique at large volumes.
The root of the problem is not the biomagnetic separation technology itself, but the bad validation and specification of the process. In addition to the buffer composition, incubation time and conditions, magnetic beads characteristics and concentration, it is necessary to correctly define the magnetic separation conditions. When parameterizing a biomagnetic separation process, we need to do something more than just define the separation time.
The first step to correctly validate the process is to focus on the relevant parameters. The basic point is to know the magnetic force that drives the beads in the suspension to the retention area. A simple permanent magnet (and almost all the classical magnetic separators) generates a magnetic field that changes with the distance. Hence, it generates a force proportional to the spatial variation of the field (technically speaking, the force depends on the gradient of the product of the magnetic moment of the bead and the applied magnetic field). This magnetic force will push the beads in one direction, which is against the drag force generated by the buffer viscosity. The magnetic bead speed is defined by the balance between both. When the same magnetic force is applied, the beads speed are lower at the higher viscosity media, i.e., the same beads would be 3-4 times slower when suspended in whole blood than when suspended in water buffers.
For standard separators, the magnetic force is highly dependent on the distance to the magnets, so its value is not the same over all the working volume. This fact may not always be noticeable at a small volume, but when the working volume scales up, farther beads will experience very low magnetic force (and move slowly) and nearest beads will experience extremely high magnetic force. As the magnetic force decreases almost exponentially with the distance, larger vessels imply that the beads need to travel longer distances moving at a much lower initial speed. To keep the recovery rates high enough to make the downstream process economically feasible, the separation time for capturing the farthest beads will also need to be exponentially longer. On top of that, the beads located initially near the retention area can quickly reach the retention area (high force implies high speed), and they will remain under high force during exponentially longer times. The consequence would be that this fraction of beads would aggregate irreversibly.
This is the most usual cause of the in-batch inconsistency issue when scaling up the magnetic separation downstream process. Notice then, that for biomagnetic protein purification, it is necessary to use separation for the capture of the beads (with the molecule of interest attached) just after the incubation. Following that, wash them several times to remove debris and impurities. Finally, after changing the buffer conditions for eluting the protein, it is necessary to add another biomagnetic separation step to capture the beads and extract the buffer containing the purified protein. Recovery rates not close to 100%, large separation times, and lack of consistency, make this technology inefficient and uneconomical for biopharmaceutical downstream processes.
However, we can use the knowledge of the physics behind the biomagnetic separation to our advantage. If the problem is the lack of spatial homogeneity of the force, we can design a biomagnetic separation system with homogenous magnetic force over all the working volume. If so, all the beads will experience the same force, independently of its initial position. Moreover, the force will be well defined (a single value for all the volumes), thus it will be easy to specify and replicate at different volumes. Since the force is constant, the separation speed will be, too. Therefore, the separation time will change proportionally to the diameter of the bottle. Applying the correct separation time at each scale makes the recovery rate the same as in small volumes. Additionally, the retention force is also the same at different volumes, thus no irreversible aggregation problems appear at larger scale if such do not exist at the starting small volume. The IVD industry has successfully used this approach for more than 10 years, allowing larger manufacturers to scale their biomagnetic separation processes up to tens of liters by coping with the growing demand of Chemiluminescent Immunoassays.
The latest generation of biomagnetic separation systems have an additional feature: process monitoring. The homogenous starting suspension is usually opaque, changing to transparent when all the magnetic beads are separated. Optically recording the changes on the suspension transparency allows the trace of each single separation process, providing a tool for objectively defining the right separation time for any new process. However, what may be the most important application of the optical monitoring is checking the batch-to-batch consistency of the process. At every step of the downstream process, the biomagnetic separation process can be recorded and parameterized and then compared with the standard validated curve. If the current batch shows a deviation from the expected behavior, an early quality warning can be issued at the current step. Thus corrective actions (or the discarding of the batch if necessary) can be implemented early on the downstream process, saving time and resources.
Biomagnetic protein purification can be easily implemented at large volumes if the separation process is correctly defined at early stages. If the right value of the magnetic force is correctly validated for the given beads and buffer, it is straightforward to scale-up the process up to tens of liters. So, when working on your protocol, choose the right magnetic bead and don’t forget to specify your magnetic force!
Visit sepmag's website to download this free guide: The Basic Guide for Monitoring Biomagnetic Separation Processes
Editor’s Note: This article is the second part of a two-part series about downstream biopharmaceutical processing efficiency. Part I can be found in Pharmaceutical Manufacturing’s 2015 Biopharmaceutical Technical Resource Guide.