In the production of monoclonal antibodies (mAbs), finding ways to remove bottlenecks and improve yields in downstream processing continues to be a key focus area for biopharma manufacturers. Downstream processing generally takes place over a period of a few weeks and involves many unit operations — including multiple chromatographic steps and filtration steps, and more than a dozen buffers and cleaning solutions as part of the process. In fact, about 60-80 percent of the total cost of producing a mAb can be attributed back to downstream processing.
During the capture step, the goal is to isolate, concentrate and stabilize the protein of interest. During this purification step, protein A has become the most widely used resin due to its highly specific nature, ease of implementation as a standard purification process, and strong regulatory track record. It is well known that the cost of the protein A capture step is substantial, and that buffer preparation is an area for improvement to help improve both cost and efficiency.
In this article, we will highlight strategies to improve the productivity of the capture step through the selection of a high-performance protein A resin and optimization of buffer preparation. Buffer preparation will be further discussed by comparing in-house buffer preparation and ready-to-use buffers, or buffer concentrates utilizing latest technologies and supply chain options.
How resin choice impacts overall operations
When choosing a protein A resin, the resin dynamic binding capacity (DBC) is a critical factor that impacts the productivity of the overall protein A process. A resin with higher DBC can improve the productivity of the capture step while keeping the column sizes the same and minimizing the facility modification, specifically for high titer cell culture processes.
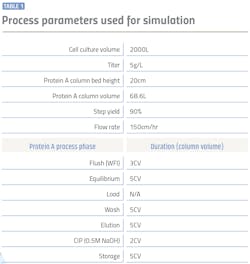
A simulation was performed with BioSolve software using three model resins having binding capacities ranging from 30g/L to 65g/L to calculate the number of bind/elute cycles, process time and amount of buffers required for 2000L bioreactor batch. Assumptions made for the calculations are summarized in Table 1 where the column size was kept consistent as 68.6L for 2000L cell culture reactor with 5g/L titer value. Productivity of the process was evaluated in terms of number of cycles required per batch and process time.
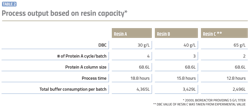
Resins having higher DBC significantly reduce the number of cycles and total downstream processing time as illustrated in Table 2. In addition to increasing productivity of the process, reduced number of cycles allows for reduced operational risk and lower labor and consumables cost for each cycle.
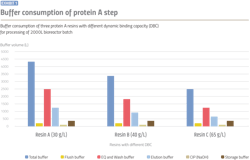
A lower volume of buffer consumption not only impacts raw material cost, but buffer preparation time, buffer tank size and method of preparation. In this model, total buffer consumption was reduced by approximately 30 percent with the use of resin with high DBC, which was resin C when compared to resin B, and reduced by approximately 40 percent when compared to resin A. Lower buffer solution requirements also provide flexibility to either make buffers in-house or utilize ready to use buffers.
Buffer preparation options
Buffers for the protein A chromatography step can be supplied to a chromatography skid in multiple ways. An established method to generate buffers in-house is with the use of WFI (water for injection)- grade water to hydrate powders in stainless steel tanks. This approach is suitable when large amounts of buffer are needed.
While these methods are established and ideal for large volumes, these operations need significant infrastructure, including warehouse space for holding raw materials prior to their use and a weighing and dispensing area for raw materials. The footprint for the stainless steel tanks themselves within the facility must also be considered.
New developments in single-use technology have added flexibility in buffer preparation methods, allowing small- and medium-scale facilities to move to single-use tanks for buffer preparation. This has enabled faster changeovers and cleanouts in buffer preparation, saving both time and cost in manufacturing processes.
Choosing a hybrid buffer preparation approach
A hybrid approach using both in-house systems and outsourced buffers can streamline downstream purification unit operations significantly. Additionally, the use of a protein A resin with a high DBC can reduce buffer usage to a more manageable level and the use of in-line dilution (ILD) systems will make the productions of critical buffer components more efficient. Here are some of the keys to this hybrid approach:
- The cleaning buffer, usually a fixed normality of NaOH, can be prepared in-house using concentrate or can be purchased as a 1X concentration due to the smaller volumes used to reduce safety concerns.
- The storage buffer (such as 20 percent ethanol) can also be managed in-house in the same way as described above due to low, consistent volumes that are typically required in the process, irrespective of the resin DBC.
- Volumes required of equilibration buffers and wash buffers (examples: 1X PBS or 50 mM Tris, pH 7), significantly decrease with increase in resin DBC as shown in Exhibit 1. Preparing these buffers using either in-house or single-use systems cause several operation challenges at lower DBC values due to high volume. For such buffers, the use of ILD systems using multicomponent concentrates (ex. 10X PBS) can provide operational advantages including facility footprint reduction, reduction in raw material management and availability of buffer on demand.
- Elution buffers (ex. 0.1M acetate buffer, pH 3.) usage can also be streamlined through the use of in-line dilution.
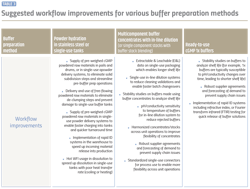
Workflow improvements for buffer preparation
Different options of a buffer prep system/process for downstream purification can be broadly split into three groups:
- Powder hydration in fixed stainless-steel tanks or single-use buffer prep reactors
- Multicomponent buffer concentrates with in-line dilution (or single component stocks with buffer stock blending)
- Ready to use cGMP 1X buffers
Industry organizations, including the BioPhorum Operations Group (BPOG) have offered insight into how buffer stock blending and in-line dilution enable overall improvements across unit operations. The decision to select one option over the other (or a hybrid approach) will usually be dependent upon an economic analysis of items such as scale, batches of drug produced per year, raw materials used and other site attributes.
Workflow improvements which can be implemented for each of the options are listed in Table 3.
Conclusion
We have shown how the flexibility and productivity of the mAb capture process step can be improved by utilizing high-capacity affinity resin along with optimal buffer management. High-capacity resin reduces the process time by allowing less numbers of cycles required per batch, which results in process cost savings, reduced risk and labor costs. Moreover, implementation of high DBC resin decreases the volume of process buffer significantly. This reduced buffer volume provides flexibility to adopt different buffer preparation processes based on the facility requirements. Based on the volume of each buffer, combination of 1) in-house hydration, 2) in-line dilution of buffer concentrate or 3) ready-to-use buffer can be applied to the process. Each facility and downstream process has unique requirements and bottlenecks. It is important to have a flexible process optimization options so that unique solution can be applied to various mAb products.