Excipients fulfill numerous functions in pharmaceutical manufacturing and are crucial to successful drug delivery in the body. The excipient family comprises a wide range of compounds with diverse chemical structures and mechanisms of action: they act as bulking agents, enhance absorption of active ingredients, prevent denaturation over a product’s expected shelf life, and much more.
Because of this functional diversity, excipients are used in a multitude of pharmaceutical products with different effects for varying purposes. However, this very same diversity presents unique challenges when it comes to laboratory analysis.
Diverse substances bring diverse challenges
Pharmaceutical companies identify and characterize excipients using a range of spectroscopic, microscopic, chromatographic and electrophoretic techniques. However, no single technique delivers ideal results for the chemical analysis of all excipients: even if one excipient responds well to a given detection method, another may respond weakly or not at all.
Additionally, some excipients require multiple or more complex methods of analysis. For instance, while excipients are largely chemically inert, they may contain low levels of reactive impurities that must be removed from the formulation, as these can affect the quality or stability of the resulting product.1 However, impurity structures are typically hydrophilic and ionic, requiring special and advanced separation techniques, such as mixed-mode chromatography. Furthermore, commonly used optical detectors require that the molecule being analyzed must have a chromophore — but some analytes lack chromophores, and thus cannot be identified via typical methods.
Despite this, the pharmaceutical industry must be able to reliably characterize many classes of excipient in order to introduce effective products to market that conform to regulations and standards. Choosing the most appropriate excipient for a given application can also bring numerous benefits for both manufacturers and patients. For instance, if a single excipient fulfills multiple functions within a formula this may reduce costs and streamline production, while excipients that bring a pleasant taste or mouthfeel to a medicine can improve patient experience.
Innovative new methods of excipient analysis
Advances in analytical technology and methodology offer innovative ways for scientists to overcome the challenges of excipient analysis, supporting the manufacture of therapeutics suitable for clinical use. Such advances include high-performance liquid chromatography (HPLC) and charged aerosol detector (CAD) systems.
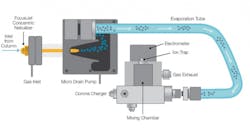
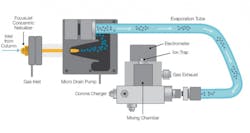
Figure 1: Schematic of charged aerosol detection (CAD) technology.
Combining HLPC and CAD has proven to be an effective way to separate and characterize excipients. HPLC facilitates a wide range of bonded phases, particle sizes and morphologies, and column dimensions, offering a flexible and robust way to separate complex samples. CAD technology, meanwhile, is more sensitive and precise than methods based on light scattering or refractive indices, is simpler to use than mass spectrometry, and can detect both non-volatile and semi-volatile analytes.2 Furthermore, CAD response for all non-volatile species is independent of the analyte’s chemical structure and uniform, single calibrant quantification can be used, so certified analytical standards are not required for analysis of known and unknown compounds in a sample.3 HPLC and CAD systems enable researchers to efficiently characterize numerous classes of excipients.
Simultaneous separation of proteins and excipients
In one study, researchers used HPLC with CAD to successfully separate and detect therapeutic proteins and amino acids, simultaneously.4 Protein therapeutics degrade and aggregate during storage, and so must be mixed with amino acid excipients to stabilize, bulk and buffer the formulation. Detecting and analyzing the most appropriate excipients for this function is, therefore, crucial. However, analyzing such a formulation usually requires active proteins and associated excipients to be measured separately, increasing the time, cost and labor involved in analysis.
Using a Thermo Scientific UHPLC system with diode array detection and universal CAD, researchers were able to reduce the time spent preparing samples and performing analysis by measuring both proteins and excipients simultaneously. The system was able to measure compounds both with a chromophore (via UV) and without (via CAD), extending the range of analytes measured in a single sample and overcoming this limitation of other methods.
Sensitive, uniform quantification of adjuvant behavior
Another study5 used UHPLC to quickly and sensitively measure the purity of immunological adjuvants—substances that help promote the effectiveness of a vaccine by reducing the amount or frequency of the required dose, prolonging the duration of immunological memory or boosting immune response. Quantifying adjuvant strength, purity, stability and degradation is subject to rigorous standards and regulations, and many adjuvants under investigation contain components that lack chromophores required for traditional methods of analysis.
During the study, scientists utilized a UHPLC-CAD system. While non-volatile compounds often show a poor response to excipient detection techniques, the researchers found that CAD was able to uniformly identify a structurally diverse range of compounds, including saponins, cholesterol and phospholipids. The team was able to measure intact adjuvant species, degradation products and potential impurities with improved efficiency and sensitivity, even in the absence of pure primary standards.
Towards safe, effective, informed preparations
Results are of the utmost importance in pharmaceutical manufacturing. To safely and efficiently manufacture products in compliance with regulations and standards, the industry needs reliable, sensitive, accurate methods to identify and analyze any excipient, regardless of its properties or behavior.
New analytical solutions are improving pharmaceutical workflows by overcoming the challenges of excipient analysis. Systems based on UHPLC and CAD can quickly and precisely measure adjuvants and impurities, and simultaneously characterize compounds both with and without chromophores. Such reliable, easy-to-use methods support manufacturers in producing safe, effective preparations with guaranteed physicochemical and biopharmaceutical properties.