As cell and gene therapy makes inroads in the life sciences marketplace, an important existing concept from this space has begun to draw more attention than it had previously: traceability.
Successful use of cell and gene therapy approaches — like chimeric antigen receptor cell therapy treatment — requires very close monitoring and tracking of sensitive personal information, sample materials, therapy processing steps, transportation, handling and storage logistics, and overall scheduling and planning to fit the treatment window. All these can tie together into traceability to ensure product efficacy.
While some of these specific needs are unique to the cell and gene therapy and personalized medicine space, the overall traceability concepts are not new and are applicable to many, if not all, current treatment styles. As a new monoclonal antibody product travels across the development and manufacturing pipeline stages from research and development through clinical trials and into large-scale manufacturing, lack of traceability in both the process definition and the associated product data can become a bottleneck to speed to market at nearly any stage.
While most organizations have long since moved away from physical paper records in favor of electronic documents, those electronic documents are still often created, stored and managed ad-hoc. This is done using a wide variety of different systems, software and formats, all driven by people sharing and moving them across the development pipeline.
When any segment of the overall tech transfer team wants to trace forward to ensure accuracy and standardization, or trace backward to identify and resolve silos of data that make the process complex, convoluted and time-consuming. Fortunately, modern digital solutions to these problems exist and can build in traceability concepts across the front end of the development pipeline through the back end commercialized space — including the supporting data infrastructure.
Traceability in recipe definition
Carefully developing and managing the master recipe for a therapy will always be critical to success. Teams need to ensure the recipe is available and traceable across all development stages. This starts with capturing the team’s product knowledge and the manufacturing process specifications during the process development stages. The next step is quickly finding a way to deliver that information thoroughly and efficiently to subsequent clinical and commercial manufacturing stages and facilities.
At every stage of this process, a significant hurdle is technology transfer — the movement of ideas, data and evolving specifications from one group to the next, often scaling up or out along the way. In any given treatment’s development life cycle, the team is likely to perform technology transfer multiple times within the development stages, and then again in the more complex transition from development to full-scale manufacturing.
As the therapy is developed and evolved, each team working on a given development stage is likely to make changes to the process sequence, equipment, procedures, cleaning and sterilization, required materials, and manufacturing scale — all of which impact the master recipe. This means teams need a good way to ensure they have clear records of which versions of a recipe they used in each stage for each batch.
Traceability in ‘what happened’
Managing the manufacturing specification and master recipe is only half the battle. It is also critically important that organizations can clearly, quickly and comprehensively trace what happened in each production batch across each development stage, including all findings. These include what worked and what didn’t, as well as feedback on what happened. All recipe elements — process sequence, materials, equipment, sampling and testing, etc. — contain key data needed for both formal records and troubleshooting problems.
Organizations need to clearly, quickly and comprehensively trace the materials that go into a production batch at all stages. While the most important variables to trace are what materials were used and how much of each material went into a batch, references and traceability do not stop there. Sourcing of materials, expiration dates, the storage process and preparation steps are all critical variables.
Before a production batch is started, the development or operations team needs to ensure that all materials are up to quality standards. Keeping this information current during use is required. If material exceptions occur from the supplier or due to other non-batch related activities, the team will quickly be able to see where else the material was consumed. This will allow them to quarantine those lots against the deviation until the issue is investigated and the potential quality impact is resolved.
As a team readies to release a batch for clinical trials or commercial market use, they will need to confirm that any material exceptions were properly handled, and that there are no open deviations, sterility concerns or other potential reasons the organization might need to quarantine the batch. Performing these evaluations during individual batch production and then having the information available for post-production investigations are all part of normal regulated manufacturing.
Scaling out traceability
When a production team in a development stage finally has a working master recipe, the need for traceability is still not complete. If a treatment starts showing immediate success during trials, or if a broader indication for a larger patient population is under consideration, an organization will need clear, traceable records for the scale-up or scale-out of production.
As treatments become more personalized, like those in cell and gene therapy, it will become more and more important to produce products closer to patient populations. This will require certification that all the local therapy sites are using the approved process recipe version everywhere and have records demonstrating that the approved version was correctly followed.
Manufacturing of treatments with broader patient populations go the other way and need larger manufacturing capacities as the treatment has more success. If an organization is also scaling its production across the globe, it will need to ensure it has a recipe that is consistent across all locations. To do this, the organization will need to carefully manage recipe versions, including differences in languages, available materials and regulatory requirements. And once execution begins, staff will need to capture material use and overall records in each of those places and then store the records to meet any traceability requirements, either immediately or in the future.
Historic organization is problematic
Today, management of most records and recipes is accomplished with scattered electronic files, technology transfer reports and batch records. In many cases, each group handling different stages of production uses different systems and different methods for defining and recording their activities. Some might be using proprietary software designed for their specific roles, while others might be keeping track of data and changes in a series of applications stored across various personal computers, servers or other protected storage locations.
To perform consolidation and validation, teams must first find all the data, which often requires reaching out to many different people and collecting many different formats of data. Sometimes the data must be converted into a format useable by the next system, which presents an opportunity for data entry errors, fostering the need for another round of cumbersome verification steps. And if a mistake is discovered — for example, if someone entered an incorrect parameter — tracing that error back to its source, fixing the problem and following up with change control is difficult and time-consuming. Each of these steps adds time that further pushes out the date of product launch, while adding complications that make it hard to see the big picture of a product’s life cycle.
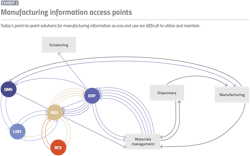
Often, such an infrastructure can have hundreds of connections. For example, if the quality team connects to the laboratory information management system to find a result from a particular product run, someone needs to make sure there are proper interfaces with all other necessary systems to ensure the production run is connected to the right sample, correct manufacturing order, resulting quality and electronic batch record data, etc. Not only is it difficult to maintain this complex web of tools, but as soon as any single element changes, there is a risk it will break something as critical connections become dead ends. Such a system must be well documented and validated. And often, when the person who built the system leaves, the team must decipher the documentation and configuration (Exhibit 2).
The wrong digital tool for the job
Since technology transfer is an enterprise-wide business process, many organizations attempt to apply their current enterprise digital tool set, such as an enterprise resource planning (ERP) system for detailed materials traceability. While several needed elements may exist in the ERP system, getting down into the details can often be messy, complex and time-consuming. An ERP system does not typically contain all the intermediate and specific manufacturing materials consumption details. Moreover, other information is maintained in fit-for-purpose solutions, typically associated with the shop floor. Navigating this complex spider web of systems requires significant time and effort, again opening the door for errors.
Fit-for-purpose tools
Modern digital technologies are built to automate traceability steps to streamline the recipe management and technology transfer stages, and to support easier scale up and scale out of manufacturing across a treatment’s life cycle. Using a tool like Process and Knowledge Management (PKM) software, a team has access to an electronic repository that captures every change made to the manufacturing specifications across the development pipeline, from research to development to manufacturing.
With PKM, every stage of the production pipeline uses the same web-based electronic tools to define the process recipe and to standardize manufacturing objects and data, improving accessibility and collaboration. Any authorized user can more easily create, locate, share and comprehend a therapy’s manufacturing specification data using fit-for-purpose generation and discovery tools at any stage in a product’s life cycle.
PKM software typically includes built-in templates and automated electronic workflows to help teams create common definitions, and to then keep them current throughout development and manufacturing. Additionally, built-in recipe management helps ensure any team member at any stage of the process is always using the best and most current version of a recipe, regardless of scale or location changes.
Master recipe management capabilities in the most advanced PKM software push changes to all recipes simultaneously, eliminating time-consuming and error prone manual data entry. And because built-in auditing automatically monitors and confirms changes, teams no longer need to spend as much time and energy on change management and associated compliance activities.
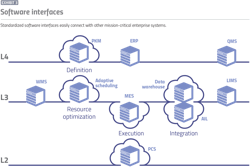
Complimenting the manufacturing specification and recipe management traceability requirements are a modern digital manufacturing operations management (MOM) infrastructure. MOM solutions incorporate key elements of today’s manufacturing execution systems, process control systems, and historical process information management systems. MOM software connects the key manufacturing, quality and demand planning systems together to create a ‘single source of truth’ by consolidating data for easier reference and management. It also provides the core manufacturing execution and data collection details. Electronic recipe execution along with digital data capture of all related manufacturing steps, equipment performance parameters, material consumption and transformation are all incorporated into this framework (Exhibit 3).
Today that means layering manufacturing automation systems on a manufacturing integration and data core. The recipe management element communicates with the PKM system to ensure use of the current version of the recipe. The electronic recipe execution element and associated electronic batch record capture is maintained within the facility’s four walls. Whenever the facility consumes a material on the shop floor, the software connects it to the batch, making it easily discoverable during and after production.
The operations and quality control teams now have shop-floor visibility of materials use and history, as well as the ability to generate traceability. This includes a ‘lot genealogy’ that enables the user to look forward to see what batches have consumed a given lot of material, or back to identify the full list of all lots of materials consumed by a given batch.
Traceability across every stage
Whether a pharma organization needs to operate in multiple world areas, or simply wants to start small and scale over time, it should adopt fit-for-purpose tools to support traceability early in the development pipeline.
This will help companies streamline manufacturing and increase speed-to-market, and will also support long-term sustainability of product delivery by helping eliminate disruptions and speeding troubleshooting. Digital solutions provide the infrastructure needed to supply staff with constant access to data for better collaboration and improved operational excellence across product development.
About the Author
Bob Lenich
Business Director, Industrial Software, Emerson
Bob Lenich is a life-long learner who stays engaged in new technology and organizational trends. In his 42 years in the industry, Bob continually aims to solve operating issues across the process industries to help Life Science manufacturing improve people’s lives. Bob has a BS in Chemical Engineering from Rose Hulman Institute of Technology and an MBA from the University of Texas.
Michalle Adkins
Director, Life Sciences Strategy, Emerson
Michalle Adkins is director life sciences strategy and direction. She loves working in the life sciences world and has done so for over 30 years. She previously led the Emerson life sciences consulting team that used their varied experiences to work with several top pharmaceutical and biotech companies to provide consulting services for digital plant maturity assessments, future direction planning, solutions mapping, business justifications, and project definition. Prior to Emerson, she worked for Merck & Co., Inc. in various capacities including instrumentation, automation, and manufacturing, as well as vaccine scheduling and planning.
Ms. Adkins has a B.S. in Chemical Engineering and an M.E. in Industrial Engineering from The Pennsylvania State University as well as a Six Sigma Black Belt Master's Certificate from Villanova University.