Moving a complex biologic out of the lab and into human trials requires a solid supply chain strategy. This guide outlines how to build that strategy, including considerations for the current COVID-19 pandemic. The ecosystem in which your drug product will operate is diverse, distributed, and complex, even under normal conditions. Success may depend on effective collaboration among all supply chain participants, both in early phase development and as the treatment volume scales. The many supply chain components outside the manufacturing facility, and the participation of the patient and health care provider, demands that there be a unique, collaborative approach to delivery, risk management and more.
Step 1: Build your strategy
There are many decisions to be made when moving a complex biologic out of the lab into human trials. In light of COVID-19, you may wish to consult the most recent FDA guidance on clinical trials. Before decisions can be rendered, it is critical that your team have a solid strategic foundation for your supply chain. Consider the following:
Does your current team have the necessary members?
Personalized therapies require a team of specialists in multiple disciplines. Establish these three roles from the beginning.
- The cell and gene specific supply chain lead should have a deep understanding of specialized couriers, “cold chain” shipping logistics and product monitoring. This expertise will be key when the team looks to move the product out of the bench lab setting and into the clinical trial ecosystem.
- The manufacturing lead is a linchpin for how the product moves through product development, capable of establishing the best manufacturing model and facility with expertise in scaling the model and staying in sync with each separate supply chain.
- The clinical operations lead must leverage relationships with clinical sites to develop proper procedures for treatment. These leads must be able to qualify clinical sites based on whether each offers capabilities required for product handling and treatment.
Has the leadership team agreed upon the operational goals and roadmap?
There are two components to operational planning — set-up and scaling — that must address the following points:
Setup
When do we want to begin enrollment? Take into account protocol development, regulatory filings/approval and IRB.
When do we foresee filing an IND? Have ongoing conversation to define and adjust trial pace and supply chain dependencies.
Have we outlined all steps in the workflow? This process mapping requires detailed cross-functional collaboration.
Scale
Is the treatment repeatable and controllable? Evaluate if the complexity of the process will remain manageable as the trial scales and identify areas for improvement, if necessary.
Is the product journey reliable? Evaluate the efficiency and reliability of the end-to-end process, and whether it can support high success rates for each product. This evaluation will likely be ongoing as your trial scales.
Is the necessary capacity available throughout the workflow? Establish that essential elements, including raw materials, are “always ready.”
Have outsourcing decisions been made? Each internal functional team needs to be in agreement about actions to be handled in-house, and which parts of the process can be handled by external partners. This should be considered across the following operational areas: manufacturing, clinical operations, and software and IT.
Step 2: Establish your operational ecosystem
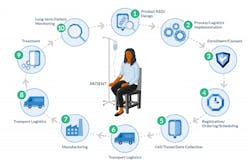
The ecosystem in which your product will operate has diverse participants and geographies, and you need to provide air-traffic control. Effective collaboration will be a critical success factor, both in early-phase development and as the treatment volume scales. Here are the participants who typically need support:
Patient material collection sites: Since medical centers are the key access points for patients receiving your cell and gene therapies, the clinical site partners you select must be a good fit between your therapy and the hospitals' capabilities.
Identify how many sites will be needed, where they will be located, and what resources already exist there to support the therapy’s workflow. In most cases, the prescribing physician and the care coordinators represent different participants than the principals in the collection process itself who are apheresis staff and/or surgical teams. This means that even with a particular medical center, multiple departments and users will be engaged. During the COVID-19 pandemic, it is also important to understand a medical center’s capacity for operating clinical trials, as well as increased patient safety protocols.
Manufacturing
Manufacturing strategy also affects your supply chain planning. Here are some key areas to consider, with flexibility gaining increased importance in the era of COVID-19:
Contract manufacturing or in-house? Both options require supply chain integration and traceability throughout the manufacturing process. Some contract manufacturing organizations (CMOs) have pre-built integrations to existing supply chain solutions.
Single-site or multiple-site manufacturing? For single-site manufacturing, your supply chain operations must be optimized to succeed at your chosen manufacturing location. For multiple-site manufacturing, you'll need to focus on supply chain flexibility giving you the ability to pivot at short notice.
Single region or global? Sometimes it will be necessary to go global earlier than anticipated, requiring a corresponding extension in supply chain operations, such as new regulatory, distribution, and language requirements.
Third-party logistics
Evaluate how each personalized drug product will move amongst the various stakeholder locations, from collection sites to inventory, manufacturing, and infusion locations. In almost all cases, a successful product journey requires highly controlled transportation services. The right partnership with specialized couriers is essential, especially during COVID-19, when supply chains are especially unpredictable. As with CMOs, some logistics providers offer pre-existing integrations with supply chain solutions.
Storage sites
Your treatment may require storage or an intermediate depot within the workflow. Ask yourself the following:
Will you house final drug products in inventory? Take into account the proximity of your inventory to the treatment centers and the product’s condition requirements.
Is the treatment site capable of storing your therapy in its required condition for as long as needed? Not all depots can accommodate advanced therapies. Find one capable of storing and meeting your therapies' required condition until patient infusion.
Technology
Starting up your digital ecosystem early will be a significant factor in the success of real-world process optimization. Implementing key digital systems early enables management of your ecosystem participants, ensures traceability, provides reliable access to critical data, and efficient scale.
Use this list to identify which of the following typical stakeholders will be involved in your processes.
Apheresis Nurse
Bag/Kit Coordinator
Caretaker(s)
Case Manager/Study Coordinator
Cell Pharmacist
Courier Manager
Customs Officials
Delivery Truck Drivers
HCP Scheduler
Hospital Administrator(s)
Hospital Onboarder
Hospital Quality Manager
Hospital Storage Manager
Infusion Nurse
Manufacturing Capacity Manager
Manufacturing Facility Manager
Manufacturing Lab Tech
Manufacturing Materials Manager
Manufacturing Quality Manager
Medical Monitor
Quality Manager
Qualified Person (if in the EU)
Pilot and Flight Crew
Primary HCP
Principal Investigator (clinical trial)
Project Manager
Refrigerated Container Coordinator
Supply Chain Manager
TSA Personnel
Step 3: Create your process
Establish processes that will provide streamlined communications and operations across all ecosystem participants. To help identify and prioritize process requirements, ask the following:
Is your dose fully established? Dosing a personalized medicine is more dynamic than a traditional one. Depending on the mode of action (e.g. an active antigenic response through gene editing) of the product and other sequenced investigational products (e.g. recombinant antibodies or small molecule products), there may be a need for several small clinical trials early in development to establish an efficacious dose while attempting to identify side effects.
Is your collection protocol established? Until the industry implements greater standardization, you will have to provide a significant amount of detailed documentation to healthcare partners to ensure consistency and quality. It is critical to work through and document each and every dependency around the collection process and care requirements for the materials, from collection to shipment.
Are your drug products going to be frozen? Whether to freeze a manufactured cell or gene therapy is a major strategic decision with benefits and trade-offs. The management team must agree upon the preferred set of upsides, and plan in detail how to address the challenges associated with each choice.Freezing can significantly reduce the risks associated with short shelf-life of the bioactive products, but comes with logistical challenges related to exact temperature-controlled storage, defrosting procedures, and management of storage devices (LN2 dewars) within medical center pharmacies. Never-frozen products are under greater time pressure, but also forego the additional steps in the supply chain required for freezing and defrosting.
Has everyone agreed on the trackable data within the chain of identity (COI) and chain of custody (COC)? The COI and COC must capture a multitude of different data types in a detailed and consistent format to satisfy the requirements by the FDA and EMA. Your process should define how to collect and manage data within a single record and ensure the information is accessible to regulatory oversight on-demand.
It is critical to identify the required data upfront as your therapy moves from the research lab to human trial. Tracking COI/COC is required by the FDA and EMA. It is critical that this data be identified and detailed early on, be format-consistent, and be report- and audit-ready at any time, even in early phases.
Is your labeling plan established? When it comes to your supply chain, the product label is of utmost importance to guarantee that the product is flowing through the process correctly. Here are high-level examples of the key factors that will determine a complete view of your COI and COC.
How the product will be labeled: One of the most challenging process specifics to nail down in practice, this will determine where the label will be printed (at your facility, at the facility of a third-party partner such as a CDMO, or the collection site) and who will do the printing (your team, a third-party partner team, or HCP staff).
What goes on the label: Aim to include the regulatory-required information, plus any other critical information, without overloading the label. Products that will be shipped regionally or internationally may need to address language differences.
How the label looks: The information must be easily read and utilized in order to prevent product mix-ups at any point in the supply chain and meet various regulatory needs (which can vary country by country).
While not explicitly designed to cover the personalized therapeutic process, existing labeling standards, including ISBT 128 and Single European Code, can provide a starting point.1
Step 4: Develop your Quality Risk Management program
Quality Risk Management (QRM) has become exponentially more important with the introduction of advanced therapies. The FDA defines Quality Risk Management as “a systematic process for the assessment, control, communication, and review of risks to the quality of the drug product across the product lifecycle.”2 Compared to traditional pharmaceuticals, the complexity of the manufacturing process and supply chain for personalized therapies creates new opportunities for deviations and issues to arise.
Patient safety is the key driver for QRM. QRM is defined, expected, and used by regulators focused on this cornerstone of any approval process. A robust program can also provide a number of additional benefits, such as to help ensure a high-quality drug product, consistent compliance, operational efficiency, and improved decision-making in the event of an issue. When effective, QRM can also help reduce time to correct issues or deviations, increase customer service for patients and health care providers, and lower costs through improved operations and fewer failures.
The QRM process
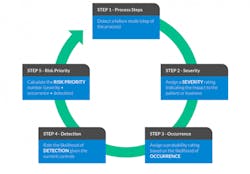
The industry methodology most commonly used and proven effective is “Failure Mode Effects Analysis” (FMEA), a quantitative process to help participants assess the different ways each step, or mode, of the end-to-end value chain can fail, what the effects of a failure might be, and which risks are the highest priority for avoidance and mitigation strategies.
The FMEA process
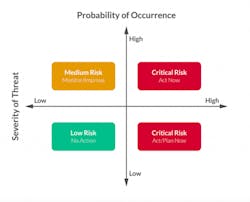
The result of the FMEA process should provide a clear map of what areas need the most attention to reduce overall risk (as seen in the diagram below) and prioritize what could otherwise seem like an overwhelming amount of work. The process also lays the foundation for the internal and external stakeholders to move forward.
Prioritizing risk assessment results
Who is responsible?
The owner of the risk assessment process can vary among biopharmaceutical sponsors. Most commonly, staff from the Quality or Technical Operations group take the lead, but more mature companies may create a dedicated group or function that specializes in assessment and improvement activities.
QRM is a continuous effort, and the risk assessment process should be revisited at various points in product development, clinical trial phases, and pre/post-approval. Information and learnings will grow across the value chain as data is collected from ongoing operations and stakeholders gain more experience with the product, processes and patients.
Conclusion
With more than 1,050 advanced therapy clinical trials underway, every new drug product process represents a unique set of supply chain challenges.3 This is the time to set up your supply chain for success from the start, especially during a time of increased variability. Advanced therapies must always operate in an environment of some unpredictability, and in the era of COVID-19, a successful strategy will address volatility up front. The good news is that developers for cell therapy, gene therapy, and personalized cancer vaccines are no strangers to the unexpected. Experienced leaders in this field know that scientific progress depends on a well-prepared process. Effective management of four key supply chain components — strategy, operational ecosystem, drug product process, and Quality Risk Management — will establish a solid foundation for early learnings and eventual scale.
When a patient is the process and the product, safe and effective personalized therapeutics start with proactive supply chain readiness.
References
1. ICCBBA. 2019.ISCT-128: Cellular Therapy. http://www.iccbba.org/subject-area/cellular-therapy
2. FDA/CDER/CBER Guidance for Industry. Q9 Quality Risk Management, IV General Quality Risk Management Process, p.3. ICH, June 2006.
3. Alliance for Regenerative Medicine. Q3 2019 Data Report. https://alliancerm.org/publication/q3-2019-data-report/