An intelligent approach to managing serialized product data
Serialized medicines are the new normal for the global pharmaceutical industry — and they are a powerful weapon in the fight against drug counterfeiting and diversion. But serialization also creates enormous complexity and new sources of supply chain disruption, making it difficult for companies to take control of their serialized operations, achieve continuous compliance, meet operational excellence goals, and unlock the true value of their serialization data. Moreover, serialization inconsistencies can have a reverberating effect well beyond the four walls of compliance teams, as these challenges can even delay or prevent the delivery of life-saving medicines to patients in need.
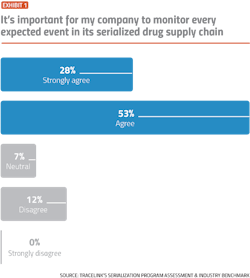
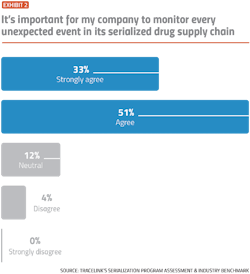
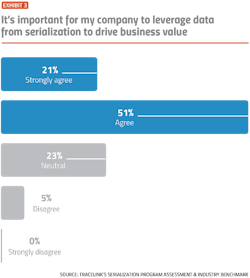
While the vast majority of pharma companies indicate a desire to be proactive and detect compliance problems early, slow-moving manual processes and a lack of visibility into serialization data are making it difficult for companies to achieve operational excellence, according to results from TraceLink’s Serialization Program Assessment and Industry Benchmark.
Threats to operational excellence often come in the form of serialization process exceptions that result in costly product delays. Failure to resolve serialization process exceptions can quickly lead to lost sales, damaged reputation, expired inventory, scrapped product and even drug shortages. As regulatory enforcement heats up, manufacturers may also be subject to hefty fines for compliance oversights that might have been prevented.
To get out of fire-fighting mode and achieve operational excellence, companies need to shift into proactive mode — consistently monitoring their serialized supply chains to accelerate the resolution of inconsistencies and leverage actionable intelligence that can help stabilize and streamline their serialized operations.
Resolving serialization exceptions
To determine the cause of a serialization exception today, serialized operations teams must manually download, combine and analyze data from various sources, including but not limited to provisioning data, commissioning data, shipments and receipts data, and compliance data. The process can take hours, days or sometimes much longer depending on the scope of the problem, the amount of data being reconciled, and the number of personnel available to address the issue.
While operations teams race to resolve exceptions, products can remain stuck somewhere along the supply route, because several regulatory markets require 100 percent serial number reconciliation before products can be released for distribution. Each minute a product shipment is held up threatens companies’ ability to ensure medicine availability and meet patient needs.
Complicating matters further, many manufacturers now offer highly specialized and personalized medicines to smaller communities of patients. These precision medicines are often fragile with exacting transportation and environmental control requirements. In addition to heavy financial losses for manufacturers, a lost or delayed shipment of precision medicine could prevent patients from receiving the right treatment at the right time. The need to protect patients by keeping products flowing safely and smoothly through the supply chain has never been greater.
The costs of supply chain disruptions
Serialization process exceptions can have an enormous financial impact — especially when they result in significant product delays or batches that must be scrapped. Additional expenditures include costs related to managing and resolving exceptions, inventory carrying costs, expired inventory costs, and expenses incurred when shipments must be returned, repackaged, relabeled and redistributed.
Product shipments that are stuck in customs or at a wholesale distributor’s warehouse or somewhere else along the supply chain, immediately begin to lose value and efficacy as they get closer to expiration dates. As a result, manufacturers may be forced to offer those products at a steep discount, if they are still viable at all. Product delays also provide opportunities for competitors to step in and gain an edge. For smaller manufacturers with limited product lines and margins, any amount of lost business or reputational damage can be a major problem.
The average disruption resulting from a serialization process exception lasted two days in 2019, although some lasted much longer, according to an analysis of pharma manufacturers on the TraceLink network. The average value of pharmaceutical products held up while serialization process exceptions were addressed is estimated to be $50,000-100,000 per incident.
Large manufacturers experienced an average of two to four exceptions that resulted in supply chain disruptions each week. Small and midsize manufacturers experienced roughly one supply chain interruption per week, or about 50 per year. These disruptions amount to millions of dollars of stuck inventory and delayed revenue recognition each year.
The global supply disruption landscape
Throwing vast sums of money at serialization process exceptions is not a sustainable strategy. With more countries enacting complex and widely varying serialization and track-and-trace regulations, the issue is only growing more complicated and increasingly expensive. Recent developments in newly regulated markets such as the European Union, Russia and Brazil illustrate just how easy it is to encounter serialization process errors, and just how challenging the problem has become for manufacturers.
The European Union’s Falsified Medicines Directive (EU FMD) requires manufacturers to upload serialization and product master data to the EU Hub, which then disseminates the information to the National Medicines Verification Organizations for European Economic Area member nations. Since EU FMD became law on Feb. 9, 2019, manufacturers have been deluged with alerts generated by the EU Hub. With EU FMD transition periods ending and more EU countries enforcing serialization regulations, compliance-related errors will increasingly lead to shipping delays.
Russia recently implemented the most complex and extensive track-and-trace regulations in the world. Manufacturers must report on a wide range of product movements, custody transfers, operational events, and packaging changes. Serialization events must be time-stamped and reported to the Russian Drug Circulation Monitoring System (MDLP). When time stamps are out of sync, the MDLP begins sending error messages that can result in supply disruptions. Manufacturers can expect Russia to ramp up compliance enforcement in 2020 and beyond.
Manufacturers conducting business in Brazil are also facing a challenging regulatory environment. The country, which began the final commercial implementation phase of its national drug control system in 2019, is requiring manufacturers to comply with a wide range of complicated serialization, aggregation and reporting requirements. The asynchronous nature of the data exchange with ANVISA, Brazil’s regulatory authority, has the potential to lead to serialization process exceptions.
The right tools for the job
Supply chain disruptions have a direct impact on companies’ ability to achieve operational excellence. Pharma manufacturers require a solution that enables them to detect potential errors early — before products leave the organization’s four walls — and mitigate the costs of supply chain exceptions. Key capabilities required to rapidly resolve exceptions include:
- Monitoring commissioned lots
- Automatically reconciling serial numbers
- Viewing serial number event histories
- Analyzing message exchanges with trade partners
- Generating audit logs of all user and system actions
Operations teams that take an automated approach with a serialization intelligence solution will be empowered with self-service capabilities that enable them to proactively identify and resolve problems faster than they can today — and without additional help from the IT department. But that’s only the first step in the journey. With the right serialization intelligence solution, operations teams can boost efficiency and achieve operational excellence; free up resources to focus on mission-critical tasks; and quickly and easily demonstrate the value of serialization investments across the company.
Using serialized data to predict the future
What if serialization data could offer precise, near real-time visibility into inventory across the supply network? Improve sales forecast accuracy? Improve a new product introduction? How about reducing losses from expiring inventory, or improving inventory allocation during a time when supply and demand are fluctuating by a big margin?
Equipped with the right data platform and analytical tools, it is possible to control costs and achieve operational excellence across compliance and serialization operations. But for product managers wanting to know more about their product’s future, they’ll need to understand how to mine serialized data for its intelligence.
As pharma manufacturers and their contract partners serialize drugs and ship them around the world, supply chains will need to be closely monitored to achieve continuous compliance and prevent supply chain disruptions that occur from the complexity of global regulations. The data to do that intelligently is available now and forward-thinking pharma manufacturers are applying serialized product data to their overall enterprise and business strategy.