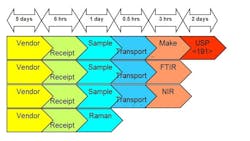
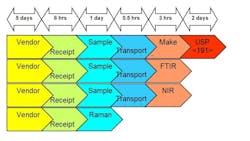
As part of an effort to Lean its raw material QC process, Lonza Biologics’ Portsmouth, New Hampshire facility evaluated several new spectroscopic technologies—Raman, NIR, and FTIR handheld or portable devices—for rapidly verifying incoming raw materials. The manufacturer sought to shave significant time off its compendial, lab-based sampling and analysis of materials, without sacrificing ID accuracy and specificity (see Figures 1 and 2 at end of article).
Ultimately, Lonza selected handheld Raman devices (TruScan, marketed by Thermo Fisher Scientific) to roll out in Portsmouth, and to extend this implementation worldwide to all of its biologics facilities.
A key factor was the need to have a more transparent supply chain and harmonized processes, says senior QC manager for raw materials, Derek Hubley. Increasingly, customers prefer materials testing and specifications to be consistent from one site to the next, he says. We spoke with Hubley about the project.
PhM: Was raw material ID an obvious candidate to be leaned, and if so, why?
D.H.: Yes, this was the most obvious candidate. Both sampling and testing are the lengthiest activities in the raw material receipt to release process. So, streamlining this portion was obvious. By decreasing the sampling and testing of raw materials, the supply chain process becomes more flexible—ultimately, allowing for a significant reduction in inventory carrying costs and raw material lead times.
PhM: Can you elaborate on why inventory carrying costs were a problem?
D.H.: Basically, everything has a lead time associated—ordering, sampling, testing, and release are all part of the chain events. So, decreasing the sampling and testing times would result in a decrease in the inventory required for the processes. For example, if we apply a three-week lead time from the vendor and a three-week lead time for sampling and testing, we would need to keep six weeks of materials on-site to support the processes. Using our new method, we can keep the three-week lead time from the vendor, but decrease the sampling and testing time to one week. This results in the need to carry only four weeks of materials on-site to support the manufacturing processes. In this case, the inventory carrying is cut by two weeks, and in the case of high-dollar materials this can be a tremendous savings per year on the total inventory carrying cost. This is one example, as the vendor lead times can change per material and the sampling and testing lead times could vary per material.
PhM: For accuracy and sensitivity, you found Raman and NIR to outperform FTIR for various materials. Can you explain this difference?
D.H.: The main reason Raman and NIR showed better accuracy was the design of the study. The study was designed to eliminate the need for sampling raw materials for identification testing. Therefore, the Raman and NIR could scan through packaging resulting in greater accuracy and sensitivity. The final conclusion of the study showed the Raman having the greatest accuracy and sensitivity and this was because of its ability to scan through multiple container types.
An exercise showing the types of materials that were active using the three technologies showed most materials in the facility were active with all three technologies. However, this would be if the analysis was done in direct contact with the material. There were a handful of materials that were active with Raman and NIR, but not FTIR.
PhM: What surprises did you encounter in terms of how any of the technologies handled specific materials (salts, sugars, solvents, etc.)?
D.H.: I guess the biggest surprise was the ability of both Raman and NIR to distinguish between hydrate forms of materials such as dextrose, sodium phosphate monobasic XH2O, and sodium phosphate dibasic XH2O. All other materials functioned as expected based on the activity of the materials in the facility based on literature review.
PhM: One of the parameters that you evaluated was the ability to create reference scans. Were all three technologies capable in this regard?
D.H.: Yes, all three technologies had the ability to create reference scans. Both Raman and FTIR only required one lot of material to create the reference scan. FTIR required direct contact with the material, which we wanted to avoid, as the ultimate goal is to limit the contact with the material. The Raman reference scan was able to be created using a single lot of material. However, to make it robust, the reference scan was created in each of the different container types in which the material could have been received into the facility. For example a reference of an amino acid (dry powder) was created in a poly bag, glass container, and a HDPE bottle; and the reference scan of Polysorbate (liquid) was created in a clear glass container and an amber glass container.
As for NIR, although the capability is there, it was not evaluated during this study. This is because it takes more than one lot of material to obtain a robust library. Based on this criterion, the Raman and FTIR were scored the highest as creating the reference scan on one lot of material, which allows the instrument to be put in use faster.
PhM: You also sought non-contact ID capabilities. What were your general conclusions regarding Raman vs. NIR vs. FTIR in terms of how they handled various container materials?
D.H.: One of the key parts of the study was to eliminate contact with the material as much as possible. The evaluation showed the Raman by far stood out from the other technologies. Its ability to provide accurate and quality identifications through multiple sample containers (e.g., poly bag, HDPE bottles, clear and amber glass containers) is what separated it from the NIR and FTIR. The NIR showed very good quality and accuracy through poly bags, but not through bottles. In addition, the film thickness did not affect the quality and accuracy of the scan for the Raman, but was an issue with the NIR. Of course the FTIR was not able to provide quality and accurate scans through containers.
PhM: Your ultimate goal is to process incoming materials with no sampling, but are there still some materials you’re testing via traditional sampling, and why has this been?
D.H.: Yes, there are still some materials that would require sampling. This is ultimately based on where the material is used in the process or if there is a critical attribute that needs to be analyzed for each shipment. For instance, microbial safety testing would always need to be performed if the material is capable of supporting microbial growth. Also, excipients require more testing even if the material is qualified for a reduced testing strategy. Excipients require 100% container ID, so the time saved by not needing to sample 100% of the containers is significant; the remaining attribute testing would be performed using the number of containers required per our statistical sampling plan.
PhM: Another goal was to consolidate data, so that you could develop your own raw material specifications and testing procedures worldwide. What challenges did this present, given the diversity of Lonza’s facilities? To what extent has this harmonization across sites been realized?
D.H.: Currently the technology is being rolled out to the Biopharmaceutical divisions. The advantage in this is that the materials across the facilities are common, making the harmonization efforts less challenging. In addition, these facilities have begun harmonizing specifications and testing procedures so the challenges were expected.
Thus far, we have approximately 10 harmonized raw material specifications, which are shared between sites in the Biopharmaceutical division of Lonza, and have deployed the Raman units to five Lonza sites.
PhM: What are the next steps/challenges in your global rollout?
D.H.: The next step for Lonza regarding global deployment of the Raman instruments is the site-by-site evaluation. This involves determining if the instrument is a fit for the materials at that site, and evaluating the benefits seen thus far at the Portsmouth facility. Currently these instruments have been deployed to five sites within Lonza. The challenges thus far are creating a system to share reference scans and harmonizing the operating SOP. The largest challenge was removed by using the one site qualification strategy.
Figure 1. Technology Evaluation at Lonza (Impact on Raw Material Process)Dry Powder (e.g. 100 containers requiring 100% ID)