Possibly the most common reason that companies fail at generating a Quality by Design (QbD)-based NDA or ANDA is that the wrong measurements are taken at the wrong place at the wrong time. All too often, a given pharma producer’s “QbD team” depends on classic techniques to build a Design Space. That means those folks may try to determine blend uniformity by stopping the blender and using a sample thief to gather samples (despite the fact that even the U.S. Food and Drug Administration believes that this is the worst way to sample bulk powders), or checking content uniformity by assaying 20 tablets with a HPLC, and so forth. However, if one wants to design good tablet-based drug-delivery systems, manufacturers need to gain a better understanding of what happens in the making of the beasties, not after-the-fact assays. “Quality by Testing” is not QbD.
Because the process of making a tablet is, for the most part, a physical exercise and not a chemical one (most of the chemistry takes place in the synthesis of the API); after-the-fact tests like HPLC, hardness, friability and dissolution tell Pharma processors literally nothing about how the powder blends, flows, granulates, compresses and is coated. For these data, one requires in-process monitors that provide real insight into the “living process” that is solid dosage form manufacture. This is where PAT comes into the picture; before a product goes into production, PAT gives us knowledge and a tool to control the process/product.
Let us assume that you or your colleagues have taken the time to actually characterize the raw materials, not merely perform USP tests on them. That is, measured the particle size distribution, the polymorphic profile, moisture situation (surface or structural), levels of crystallinity, and any other physical characteristic that may affect mixing and other steps. Ironically, the industry performs porosity and these other tests for the API, which is usually the smallest ingredient in the mix. The excipients are almost regarded as “passive” and not worth characterizing.
MEANWHILE, 30 YEARS LATER
Looking at mixing the ingredients (blend uniformity is a legal requirement and generic houses must, by law, test every batch for uniformity), we have slowly accepted near-infrared (NIR) as a reasonable test of uniformity (it only took 30 years, after all). The work that Pfizer did (over a decade ago) with Zeiss gave us the first wireless, portable NIR instrument. Several other models are also available and also have been accepted (by the FDA) in NDAs and ANDAs. The development of blend uniformity by wireless NIR was performed on products with 5%-50% API content. These levels are also quite within the wheelhouse of Raman instruments and have been the standard range of APIs for decades. There are a number of fine hand-held units on the market, easily adapted for blend uniformity work, although most published accounts are for NIR applications.
LIF FOR LOW LEVEL
However, with more potent drugs being developed and tested, the API level in the mix is often below 1%, making NIR or Raman detection (in a moving bed) problematic. A better technology for low-level API concentrations is Light-Induced Fluorescence (LIF). Until recently, LIF units had only a single wavelength operational range and induced most organic molecules fluoresce (to varying degrees). There were some specificity questions that slowed its acceptance.
With newer units (see Figure 1), tunable, multiple wavelengths and wireless operation are now available. This newer type of instrument allows the formulator/operator to tune the incident light to the excitation wavelength of a low-level API, a lubricant (often in the 0.1 to 1.0% range), or any other organic ingredient in the mix. Since not all ingredients mix at the same speed (varying densities, sizes, shapes), this specificity allows any or all components to be monitored for completeness of blend.
Another way of measuring powder characteristics (after mixing) is with an automated Powder Rheometer. Pharma has been testing the viscosity of liquids for decades with similar devices, but now the technology exists where the industry can test the interactions between particles in a powder mixture. The unit combines shear, bulk, dynamic and axial powder testing methodologies for comprehensive powder characterization. This information gives us insight into parameters such as flowability, compressibility, and may even be correlated to dissolution characteristics. This can be a boon for developing the amount of lubricant (as well as type) in a manner that is no longer “hunt and peck,” but based on actual flow characteristics.
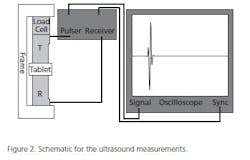
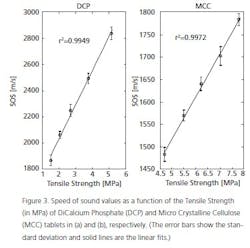
The tablet cores themselves may need to be tested prior to coating for a number of parameters: hardness, friability and tensile strength, for example. One interesting device, made to non-destructively measure parameters such as Tensile Strength (see Figure 2) of a (finished) tablet is to use ultrasonic waves. The speed with which the sound travels through the tablet is measured and correlated with standard (destructive) testing technologies. The calibration curve generated is then used to analyze process samples rapidly. The obvious advantage is the ability to measure many more doses, non-destructively. Figure 3 shows calibration curves for tablets made from DiCalcium Phosphate and Microcrystalline Cellulose. These data can be used to quickly predict physical parameters and allows formulators to understand what component ratio changes or compression changes are doing to the finished dosage form.
EXPERIENCE OF GRANULATIONS PAST
Unless direct compression, ribbon compaction, or extrusion techniques are used to make tablets, the most likely method of preparing the powder blend for compression is wet granulation. Despite what some may think, wet granulation is not just a matter of pouring a given amount of liquid into a vat of pre-blended powders. Liquid needs to be added in a specific manner, at a specific rate, and usually from a specific position in the blender. Knowing what the manner, rate and positions are is the trick to a successful granulation. Quite often, “experience of granulations past” has been relied upon for an idea of what to do.
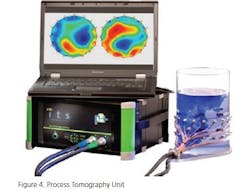
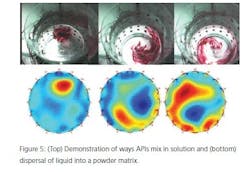
One way of measuring how a liquid disperses through a medium (powdered or another liquid) is Tomography. A series of transducers are placed around a mixing chamber (glass or metal) by measuring such things as dielectric constant changes (varying permittivity) the differences throughout a mix can be visualized, in real time. Figure 4 shows a Process Tomography unit. It is capable of measuring liquids being added into liquids (Figure 5) or dispersal of a liquid into a powder matrix. The multiple transducers allow a 3-D picture to be displayed in real time. Using these data, mixing speeds, fill levels, blade type, addition speeds, and points of addition may be optimized.
Because transducers may be applied to any size container, tablet dissolution may also be optimized. The rates of dissolution are only one piece of information; the shape and extent of the “API cloud” may be seen and sampling points set at the best positions. The effects of tablet/capsule position at the bottom of the vessel may also be seen. This leads to more specific analytical SOPs and more precise testing procedures. The precise dissolution pattern, seen in 3-D, allows for faster and better dissolution formulation.
COATED FOR DECADES
Tablets have been coated for several decades; first sugar coated, now film coated for cosmetic, identification and protection against moisture, UV and visible light. Since coatings on tablets are more than cosmetic, more attention has been paid to them in recent years. Coatings are now used to protect the API and physical make-up of the core, affect or control dissolution times, and even contain an active outside the core and, thus, need to be accurately measured and controlled.
The industry’s been using NIR in the coating pan for years to measure the amount of coatings and loss of solvents. In recent years, Pharma has seen Raman probes added to the coating control toolbox. The data generated from NIR and Raman probes has been used to determine a number of things, including spray radius, solution addition speed, drying times, pan rotation speeds and exhaust functionality. However, as with any NIR or Raman measurement, the information is about the bulk tablet bed.
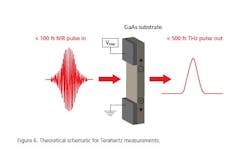
For understanding individual coating “goodness,” we now have TeraHertz spectroscopy for coating inspection. Formerly known as “far infrared,” TeraHertz has been on the scene for several years now. It may be used as simple spectroscopy, but, in my opinion, its strength is in measuring interfaces between layers. It basically sends a fast (50 fs) NIR pulse through a transducer (Figure 6) and a 50 fs TeraHertz pulse is generated. This is directed through a solid sample, e.g., a coated tablet, and some of the THz waves are reflected (first, second, etc., surface effects) and recorded (see Figure 7). The time between pulses is measured and gives the thickness of each solid phase. The pulse quality varies when there is a gap in the coating uniformity (not bonded sufficiently to the tablet, for instance).
The technique can even be used for 3-D imaging by varying the wavelengths to account for different chemicals (API and excipients, as well as coatings) within a dosage form. This is superior to NIR or Raman chemical imaging, which only shows the positioning of materials a few microns deep. THz can be manipulated to give a true 3-D picture of the dosage form.
Obviously these are but a few of the available tools for fine-tuning a dosage form as part of a successful QbD program. These and other technologies are maturing and becoming even more competitively priced and are worth careful appraisal in implementing a QbD Design Space. Not your father’s instrument package is it?
Published in the December 2013 edition of Pharmaceutical Manufacturing magazine