Design Considerations and Best Practices for Tank Vent Filtration
Many biopharmaceutical applications require vent filters—hydrophobic sterilizing-grade filters used as air vents on processing tanks. The purpose of the tank vent filter is twofold: maintain near ambient pressure in the tank while ensuring sterility in the tank. The tank vent filter removes viruses and microorganisms from the gas as it flows into or out of the tank.
To ensure proper operation and sterility, a bioreactor, for example, may have a number of vent filters including those for the tank vent, the sparge gas inlet, and the overlay gas inlet.
Understanding the diverse applications of vent filters is critical to their proper implementation and use. A number of factors must be considered in advance of vent filter implementation including filter sizing, housing and piping design, condensate control, regulatory requirements, and operational considerations such as clean- and steam-in-place (CIP, SIP) and integrity testing. Employing best practices for vent filter use can avoid common problems during installation, CIP, SIP, integrity testing, and operation.
This article will describe an application-based approach to vent filter sizing, in situ integrity testing of the filter design, best practices for housing design and vent filter operation, and a risk management approach to implementation and replacement of vent filters.
Vent Filter Sizing
Air flows in and out of a process tank commonly for two reasons: the first is to replace a volume of liquid as it is pumped in or out of the tank. Sizing the tank vent filter for pump-out or fill rate is relatively simple as the air flow rate will be equal to the pump-out or fill rate. With flow rate and pressure determined, a flow/pressure change (ÄP) curve can be used to determine sizing.
The second reason air will flow into a process tank is to compensate for the volume change associated with steam condensation. At the end of a tank SIP procedure, steam in the tank will cool and undergo a phase change to liquid water. There is more than a 1,000x difference in volume between an amount of water in gas phase vs. liquid phase. During cooling, sterilized ambient air must be allowed into the tank to prevent vacuum. Sizing the vent filter for steam collapse requires knowing the vacuum rating of the tank and the convective cooling rate. These can be calculated based on the tank dimensions including height, diameter, and wall thickness. At EMD Millipore, we have developed a computer program to facilitate these calculations.
Improper tank vent sizing can result in low pump-out rates, loss of sterility due to a ruptured disk or filter failure, or worst case, tank implosion. Fortunately, proper sizing is not difficult as long as the flow requirements and driving force are understood.
Tank venting can be static or dynamic with each requiring filters that are sized slightly differently. For static venting, the air outside of the tank is assumed to be at ambient pressure, so the driving force for airflow is determine by the pressure difference between the inside of the tank and the atmosphere. For dynamic venting, compressed air is fed to the tank in order to minimize any difference in pressure that occurs between the tank and atmosphere.
Static tank venting is commonly used for buffer tanks and intermediate storage tanks. To determine the proper size for a static tank vent filter, we utilize a four-step process:
- Determine the maximum flow rate for venting that the vent filter will need to provide. This will be either the process flow rate or steam collapse rate after an SIP cycle.
- Select the maximum pressure drop that you want the filter to experience. The pressure drop is typically less than 5 psi and should be dictated by the vacuum rating for the tank or rupture disk vacuum rating. Clearly, it is important to avoid pulling a strong vacuum on the tank that might cause collapse.
- Calculate the number of filters or filter area required to meet flow rate and pressure drop requirements.
- Ensure an adequate safety factor (~1.5x) and select the appropriate filter configuration for the tank or application.
Table 1. Vent sizing process for a static vent.
Common uses for dynamic tank venting include bioreactors and other applications where steam is replaced with compressed air after an SIP cycle. In this case, the process for sizing a filter varies slightly from the static model and is as follows:
- Select the desired pressure drop. Pressure drop is typically less than or equal to 2 psi, especially when calculating for bioreactors where minimizing vacuum is key to maintaining a sterile environment in the tank.
- Calculate the air flow rate necessary to replace the steam during steam collapse post-SIP.
- Calculate the number of filters or filter area needed to meet flow rate and pressure drop requirements.
- Ensure an adequate safety factor (~1.5x) and select the appropriate filter configuration for the tank or application.
Dynamic vent sizing can be significantly more complex than static venting since the steam collapse rate needs to be calculated for the specific tank and process conditions being used. In this case, it is recommended that software, such as that developed by EMD Millipore, be used to calculate the proper dynamic vent filter sizing.
In Situ Integrity Testing of Filters
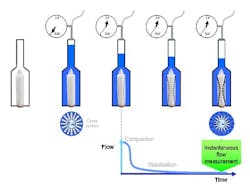
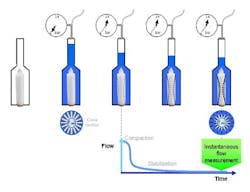
Vent filters are typically tested using the Hydrocorr water flow integrity test (Hydrocorr Validation Guide, MM document VG050).1 The test measures the resistance of the filter to water intrusion (Figure 1). A filter is placed in a stainless steel housing which is flooded with water. Under a pressure of 40 psi, there is compaction of the filter. Over time, the compaction stabilizes and the flow decreases. Once stabilization is complete, an instantaneous flow measurement can be taken; if the measurement is below the specification of the vent filter, it is considered to be integral.Housing Design and Vent Filter Operation
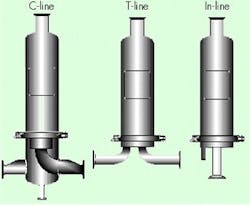
Best practices for the implementation of vent filters include selection of the proper housing. Three housing options are available (Figure 2):
- C-line. Offers the best condensate trapping capacity on the upstream side of the filter. If the stream is in an especially moist or humid environment, the C-line format allows excellent removal of condensate.
- T-line. While this format is popular, it is not optimal for vent filtration, as the housing has to be tilted for proper condensate drainage.
- In-line. This format allows the downstream condensate to drain directly into the vessel.
- Avoid pressure pulsing across the vent filter.
- Minimize a high-pressure change across the filter at high temperature as the filter can become weaker at elevated temperatures.
- Steam the vent filter in reverse direction from the tank to ensure condensate removal from the core of the filter.
- Delay the vent filter sterilization step until after the tank heating phase is complete.
- Air/condensate should be sent via the drain and not the vent filter during the heating phase.
During the operation phase, it is best to avoid fouling of the filter from entrained liquid in the tank especially when using the filter on a bioreactor. A heat jacket/blanket/trace should be used during the process if the potential exists for moisture build-up.
Risk Management
Regulatory authorities have addressed the implementation of vent filters. All advocate a risk-based approach be used to establish filter re-use and integrity testing policy.
A typical risk assessment and process validation plan should consider the criticality of the process and application of the vent filter, the number of re-use cycles, process implications, and the impact on filter lifetime.
Regulatory documents establish two types of applications for vent filters: critical and moderately critical applications. Critical applications are those in which filtered gas is in direct contact with the sterile final product. In this situation, the sterilization cycle must be validated and performed before each use of the vent filter. The filter must be integrity tested upon installation and following each use.
In moderately critical applications, the filtered gas is not in direct contact with the final product. In these applications, sterilization and integrity testing frequency should be established based on the following risk assessment parameters:
- Historical in-process filter integrity test data
- Microbiological/bioburden limits established for the filter
- Filter process conditions
It is common practice to reuse vent filters over multiple cycles. A risk-based assessment should help guide reuse and change-out criteria. The assessment should consider the following:
- Maximum number of sterilization cycles (a manufacturer or internally set limit)
- Preventative maintenance schedules for related equipment (the tank on which the vent is mounted)
- Potential for cross contamination from different product strains
- History of integrity test failures
- Humidity during operation
- Maximum pressure drop for non-clean air stream
- Service life of the filter at the temperature for heat traced housing
- Specified utilization number of uses expected for the filter
Conclusion
Vent filters are used in a number of applications, each of which can bring different requirements for proper implementation and use. Different sizing methods exist and selecting the right parameters can help ensure safe and economical use. Housing selection and orientation is important for proper operation and the application of best practices for vent filter use can avoid most common problems during CIP, SIP, and integrity testing.
An applications-based risk assessment should be used in order to properly establish vent filter integrity testing and change-out plans.
References
1. Jaenchen, R., Schubert, J., Jafari, S., and West, A. “Studies on the theoretical basis of the water intrusion test (WIT). European Journal of Parenteral Sciences, 1997, Vol. 2, No. 2, 39–45.
2. Technical report no.40 “Sterilizing filtration of gases” PDA Journal of Pharmaceutical Science and Technology, 2005, Vol. 58, No.S-1.
3. Pharmaceutical Inspection Convention, Pharmaceutical Inspection Co-Operation Scheme. Recommendation on the Validation of Aseptic Processes. Section 9.6.1, January 2011.
4. EC guide to GMP Annex 1 “Manufacturing of sterile medicinal product”, 2003.