Every pharmaceutical manufacturing plant features an area in which raw materials are weighed and transferred to clean containers. This area goes by various names, including Weighing, Weighroom, Central Weigh, Pharmacy, Dispensary, Dispensing, Fractionation and Subdivision. No matter what it’s called, its design is essential. This article outlines some best practices for design, layout and operation of these areas.
Three basic principles should drive the design of any pharmaceutical weighroom:
-
Unidirectional flow of materials and personnel
-
Segregation between hazardous and non-hazardous materials
-
Separation of storage and manufacturing items and spaces.
In the past, convenience dictated the placement of weighrooms, traditionally located right near warehouses where materials were stored. Today, the weighroom is viewed as the entry point to manufacturing and the transition point for materials coming from the warehouse and entering process areas, so specific criteria will determine the best location.
The typical weighroom is made up of three sections: raw material staging, weighing and work-in-process staging.
Weighroom design depends on the type of processing that will take place in the process area. Manufacturing operations can include: active pharmaceutical ingredients (API), solid dose forms (SDF), liquids, ointments and creams (LOC), sterile injectables (SI), and biopharmaceuticals (cell culture and fermentation).
Raw Material Staging
Raw material and work-in-process staging should be directly adjacent to the weighroom, thus reducing the requirement for transition spaces. When weighing is complete, there may still be material left in the containers received from the client. Large quantities are usually returned to the warehouse, but small quantities, especially of those materials that will be used again soon, can be stored on pallets or on shelving in raw material staging areas.
Many ingredients require special conditions for storage, such as temperature and humidity control. If the materials are to be held for any length of time in the area, appropriate measures should be taken.
Large Lot Dispensing
With APIs, SDFs and LOCs, large quantities of material can be weighed into intermediate bulk containers (IBC) of up to 2,000 liters in size. In the case of SDFs like tablets and capsules, excipients typically make up the largest percentage of raw materials. These inert ingredients, such as corn starch and lactose, are used as fillers and binding agents for the formation of tablets and the filling of capsules.
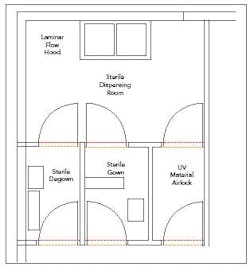
Figure 1. Sterile material weighing room layout.
The weighing equipment in these rooms is normally a pit-mounted floor scale with minimum dimensions of 48 x 48 in., or load cells mounted on a support frame or a post hoist. In either situation, powder should be transferred by gravity or vacuum.
Small and Intermediate Lot Dispensing
In the case of the remaining operations, small quantities of materials will be weighed out and placed on clean pallets to create “kits.” The kits will usually contain all of the materials required to make one batch.
Weighing in these suites is handled manually, with operators using scoops and drum-tipping devices. Scale size is determined by the load capacity and by the ergonomic capabilities of the operators. Platform floor scales that can be accessed with a hand truck are used for weighing 50- to 100-kg quantities into plastic and stainless steel drums and tubs. Platform scales can be used for pails or plastic bags of materials ranging from 10 to 50 kg. One or two bench scales will also typically be present, the first for 1- to 10-kg quantities and the second – with a much higher level of accuracy – for weighing materials from 0 to 1 kg.
Equipment Wash and Preparation
All equipment that comes in contact with materials must be cleaned to reduce the risk of cross-contamination from previously weighed materials and airborne particulates. Small items such as scoops, small containers and sample thiefs can be cleaned in a specifically designed washroom or within the weighing room.
Larger equipment, such as pails, drums and small IBCs (holding less than 100 liters), are usually cycled through the manufacturing washroom before being returned to the weighroom. Large IBCs, particularly those above 1,000 liters, are normally cleaned with Clean-In-Place systems, in which spray balls are inserted through the top opening. A more sophisticated system involves the use of a fully automated bin washer. The IBC is placed inside the washer and the doors are sealed. The washer will open the access ports on the top and bottom of the bin and wash the inside and outside surfaces. This system is particularly beneficial when cleaning equipment used in the process of making potent compounds because it dramatically reduces operator exposure.
Specialized Suites – Potent Compounds
Potent compounds are materials that would be harmful if operators were exposed to a specified amount as defined by the Operator Exposure Limit (OEL). The OEL for each compound is set by the manufacturer and is based on the normal patient dosage compared with a Time-Weighted Average (TWA) of operator exposure during handling. A general description of containment categories follows:
• Category I: >100 mcg/m3
At this level, following normal cGMP is usually enough protection for an operator. This should include hair and shoe covers, as well as the requirement to change into a uniform that is laundered or replaced.
• Category II: <100 mcg/m3 - >20 mcg/m3This is the first category that requires the use of special equipment to create an additional separation between the operator and the materials being handled. At the Category II level, containment can usually be accomplished using laminar flow booths.
• Category III: <20 mcg/m3 - >1 mcg/m3At this point, we have reached the lower level of the capabilities of laminar flow technology and another level of control must be applied to separate the operator from the material being handled. Split butterfly valves (SBV) are usually used to meet these requirements.
• Category IV: <1 mcg/m3
Below the 1 mcg/m3 level, we have reached the guaranteed limits of SBVs and must look to isolation technology to meet this containment requirement. This includes the use of glove boxes with rapid transfer ports (RTP).
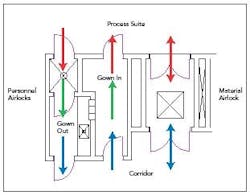
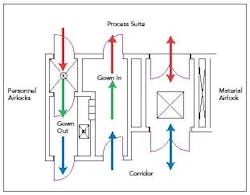
Figure 2. Typical personnel and material airlock configuration.
Low-Humidity Dispensing Room (RH <15%)
Materials that tend to be hygroscopic can be affected by moisture in the air, which can lead to clumping, poor flow and poor compression characteristics. Gelatin capsules, for example, are highly sensitive to humidity. Capsules are considered to be a raw material rather than a packaging material since the capsule becomes an integral part of the ingested product. These are weighed to ensure proper count.
In order to protect the materials, a room environment humidity is maintained by the HVAC system. Glove box isolation technology can also be used to maintain a mini-environment and control humidity within the chamber. Control can be achieved by restricting the moisture content in the airflow going into the isolator or by using an inert gas such as nitrogen.
Sterile Dispensing (Class 10k)
Materials to be used for aseptic processing may need to be received in a sterile state. In this case, special conditions must be maintained during the weighing of these materials. Typically, the environment should be maintained in the same state as the processing suite.
As in the humidity-controlled environment, this can be achieved using barrier isolation technology or HVAC controls in the room. In the latter case, an ISO 5 laminar flow hood in an ISO 7 room is typically employed. Personnel would enter the operation through airlocks from a gowning suite where they don sterile garments.
Upon request, warehouse operators pick up released raw materials and transport them from the warehouse to the weighroom raw material staging area. Any waste material generated by the pick process is collected and handled as described under the “Warehouse Waste Movement” section (p.46). The staging area may serve as the first airlock between the warehouse and the weighroom. The doors to the warehouse will be interlocked with the doors to the weighrooms, so that no two doors can be opened at the same time.
Before entering the staging area, weighroom personnel remove and bag remaining external packaging (such as cardboard, paper and plastic) and manually wipe down the material. The warehouse operators exit the airlock. Once the airlock is unoccupied, there is a recovery period to allow the particulate level to return to a steady state. Weighroom personnel enter the staging airlock, remove the material, and transfer it to the staging racks.
The weighing operator will pick up the pallet or cart from the material airlock, either manually, with a stainless steel pallet jack, or with a walkie-stacker. He will then scan the bar code on the container and transport the load to the dispensing and containing (D&C) staging area. Any unused raw material will be returned to the staging area or the warehouse.
Throughout the operation, the materials must be clearly identified, and their status and location must be recorded regularly. This can be done manually by maintaining paper documents or entering the information into a computer terminal. Increasingly, data gathering is being accomplished using bar codes and scanners and radio frequency (RF) interfaces. The data gathered from these systems can be entered automatically into the inventory control system.
Typical Weighing and Dispensing
Bulk raw materials such as lactose monohydrate are delivered to a laminar air flow (LAF) booth by an operator from the staging area. The LAF booth is equipped with floor, bench and analytical scales for weighing of majors prior to dispensing into the stainless steel drums (SSDs). Otherwise, as in the case of using IBCs, the equipment will include load cells and charge hoppers.
Drums of material are weighed on the floor scale, transferred via manual scooping to SSDs, tagged and placed on a clean plastic pallet. Pallets of bagged material will be placed inside the safe zone of the laminar flow booth and these materials will be weighed and transferred by scooping to SSDs.
The operator has received the batch record of materials and quantities to be dispensed either via a hard copy or electronically. Each weighment is recorded manually or electronically by linking the scale interface to a batch recording system.
When the weighing is complete, the LAF will be vacuumed and dry-wiped. Partial containers will be labeled, dry-wiped and palletized for storage in the dispensary staging area, or returned to the warehouse.
All ingredients for a batch will be collated as a “kit” and placed on a pallet or cart with the appropriate paperwork. The kits will then be placed in work-in-progress staging for pick-up by manufacturing personnel.
Printers may be used to print out self-adhesive labels that will be applied to all weighed containers of material. These labels may be printed with bar codes to improve material tracking.
Weighing of Hazardous Materials
For cGMPs and containment of compounds with OELs down to 20 µg/m3, properly designed LAF booths are sufficient for both product and operator safety.
When materials with OELs of less than 20 µg/m3 are handled, we require the use of transfer bottles with split butterfly valves (SBV) and glove boxes. A separate room is normally provided for this operation. The booth will be fitted with a glove box isolator (GBI) on top and a second GBI below. The GBI is equipped with an SBV for containing the transfer bottle. It will also have a port for bag-in and bag-out of drummed and bagged material that can be equipped with a drum tipper. Waste and samples will use the “sausage” or continuous bag technique for removal.
For materials below 1 µg/m3, a dispensary/subdivision glove box should be designed such that all make/break connections for raw material additions loading, sampling and product unloading are minimized and cleanable to reduce contamination risk. The GBI is equipped with a rapid transfer port (RTP) for receiving beta bags and bottles. The transfer bottle will also be equipped with an RTP. The isolator has a port for bag-in and bag-out of drummed and bagged material that can be equipped with a drum tipper. Waste and samples make use of the “sausage” or continuous bag technique for removal.
Sterile Weighing
The operator must enter the sterile weighing area via a gown-in airlock. In the gowning room, he or she will don the appropriate sterile apparel, including jumpsuit, shoe covers, hoods, gloves and goggles. A material handler will deliver the selected materials to a material airlock (MAL) equipped with ultraviolet light for the sterile weighroom (ISO 7), where the operator will pick them up.
The operator will transfer the materials to the weighing area under an ISO 7 LAF, where the materials will be weighed and double-bagged. On completion, the sterile weighing operator will manually bring the work-in-progress to the MAL, where it will be picked up by the material handler and transferred to the material staging area for pick-up by manufacturing.
On completion, the operator will sanitary-wipe the area. The scoops and labware will be placed in the MAL for pickup. The material handler will move the equipment to the wash and prep area. This process can also be accomplished by weighing out into a transfer container with an RTP, on a bench scale inside a glove box. The GBI will be equipped with an RTP for receiving beta bags and bottles. It will also have a port for bag-in and bag-out of drummed and bagged material, which can be equipped with a drum tipper. Waste and samples will also use the “sausage” or continuous bag technique for removal.
After the weighing operations are complete, the room must be sanitized. Cleaning carts and mop handles should be dedicated to the room. Wipedown of carts and mop handles with sanitizing solutions will be sufficient to leave the equipment within the areas they are used in. Mop heads, sponges, wipes and buckets are to be sanitized. Buckets will be reused, while mop heads, sponges and wipes will be disposed of as waste.
Warehouse Waste Movement
Non-hazardous solid waste (i.e., paper, cartons, plastic, etc.) generated in the weighing area is collected in plastic bins and transferred via a dumpster to a waste compactor. All contaminated solid waste should be disposed of in accordance with hazardous material regulations.
Facility Design Layout
The key element when designing a weigh facility is that the manufacturing space must, at minimum, be equivalent to conditions in the manufacturing process area. These conditions should adhere to environments where exposed product and contact surfaces apply.
All equipment must be washed to reduce the possibility of cross-contamination.
As previously stated, the weighroom should be the entryway for materials into the processing area. The ideal location is between the warehouse and the manufacturing suites. This can be the location for materials to be transitioned from warehouse pallets to in-house pallets.
Some facilities transfer materials from the wooden pallets or slip-sheets received in the truckload to plastic or metal pallets for use in warehouse storage. Even though these pallets are not wood, they should not enter the manufacturing space. In an ideal design, the “in-house” pallets will be returned to a wash area adjacent to the weighroom. The pallets are manually or automatically washed using a pallet washer and stored in a clean space, ready for use in the weighroom.
In a pharmaceutical facility, the transition usually occurs in an airlock. The purpose of an airlock is to protect the adjacent space with regard to:
- Change of air class;
- Containment of potent compounds; and
- Cross-contamination.
Key areas are the material airlock (MAL), the personnel airlock (PAL) and the gowning and degowning rooms.
Equipment Requirements
The equipment required in the dispensing suite is based on various criteria. We have already discussed equipment for materials requiring special handling. Let us now look at some of the basics.
Size of weighments will be key in selecting transfer containers and lifting mechanisms. In a typical aseptic facility, small quantities of active ingredients are involved – small enough that they can be transferred to the formulation suites in plastic bags or small containers. Excipients such as salts for buffering are also handled in small volumes. Operators will manually scoop materials from bags and drums. Here we would expect to see a 1-kg bench scale and a 20- to 50-kg floor scale.
Liquids, creams and ointments require the capability to weigh out larger volumes of material. The weighroom may be equipped with bench and floor scales as in the aseptic operation, as well as platform scales with 500 to 1,000 kg capacity. On a percentage basis, the larger volume scales are less accurate than their smaller siblings.
Solid dosage and large-volume parenterals (LVP) are typical of operations that require handling large amounts of ingredients. In the case of a solid-dosage tablet compression or capsule-filling operation, the largest portion of the ingredients to be weighed consists of excipients. In the case of solid dosage products, excipients such as corn starch and lactose can represent 99% of the tablet weight when used in potent compounds.
In an LVP facility, 90% of the products are carbohydrate or electrolyte solutions, which contain large quantities of dextrose and sodium chloride. These may be manually weighed out in the weighroom or transferred from bulk supersacks or silos.
We can expect to see the same scales as previously mentioned, plus pit scales with capacities up to 10,000 kg. In both the mid and high ranges of dosing requirements, it may be necessary to install equipment that can assist the operator in moving, lifting or dumping the containers. Vacuum-assist on a two-axis boom is used for moving drums and bags of materials. Fixed or portable post hoists can aid the operator when locating, inverting and discharging materials in containers with fixed sidewalls.
Given that the weighroom is the primary transition point from warehouse to manufacturing, it is clear how important it is to properly design and use this vital element of any pharmaceutical manufacturing facility.
About the Author
Nick Phillips has over 30 years of experience in manufacturing and engineering. For the past 11 years, he has been with CH2M Hill Lockwood Greene as the principal technologist, Manufacturing and Life Sciences, developing master plans and conceptual designs for manufacturing systems and production facilities. He has also audited pharmaceutical plants with regard to cGMP and potent compound containment. He received a BME and a MS in Management from Stevens Institute of Technology. He may be reached at [email protected].