The drug industry has often been criticized, rightly or wrongly, for its unwillingness to use new technology. Results of our 2008 survey on automation and control practices suggest that time and budget pressures may be motivating some companies to stick with the “tried and true.”
At the same time, though, more pharmaceutical manufacturing operations appear to be developing the building blocks they will need for improved control: integrating incompatible databases, connecting field-level data to the plant floor, improving data quality to allow for process modeling and optimization. Respondents also see simulation playing a more important part in daily operations, particularly in process optimization.
This year, 62 individuals in API and finished pharmaceutical manufacturing responded to our survey, offering a snapshot of industry practice, issues and preferences. Here is a very brief summary.
Among the key findings:
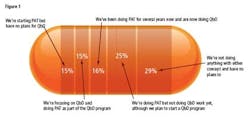
- 29% of respondents said that they had no plans to start either Process Analytical Technologies (PAT) or Quality by Design programs, compared with nearly 15% who say their organizations are doing PAT as part of a broader QbD effort, 16% who say they have focused on PAT efforts first and are now doing QbD, and15% who plan to launch PAT but not QbD (Figure 1).
- Where senior and middle management was viewed as the key impediment to progress last year, this year’s respondents pegged time pressures (44%), costs (34%) and lack of clear understanding of how to implement these programs (34%) as the drawbacks to implementing PAT and QbD. Lack of skilled personnel (27%), insufficient management support (21%) and inability to justify these efforts (21%) were also cited as drawbacks (Figure 2).
- 31% of respondents said their organizations do not routinely use process capability analysis or statistical process control. Those who use SPC are mainly using univariate methods, even though many pharmaceutical manufacturing settings are inherently multivariate.
- Paperless recordkeeping is still a ways off at most drug manufacturing facilities. Although 22% of respondents say they are using MES and 46% are planning to install MES, 29% are not.
- Process improvement and automation are becoming more closely aligned at more respondents’ companies. 46% say they’re making progress or have made this a priority and are achieving alignment, whereas 22% describe progress as stalled or nonexistent.
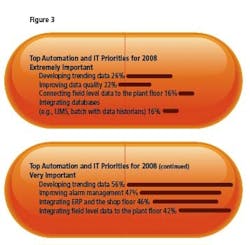
- Only 9% say they have achieved plant floor to enterprise integration. 29% say they’re almost there, and an equal number have established a basic foundation for such integration. Data Optimization and Connection: Top Priorities Developing trending data for equipment and manufacturing was cited by most respondents as the top priority, with 26% describing these efforts as “extremely important.”
Improving the quality of data to permit process modeling and optimization was the second priority, which 16% of respondents noted as extremely important. Respondents noted that other critical areas of focus are: connecting time series and relational databases, and connecting field-level sensor data to plant-floor manufacturing. Improving alarm management was another key focus (Figure 3).
Still Throwing Processes “Over the Wall”?
Responses suggest that manufacturing and R&D teams are not communicating early in the drug development cycle. Such connection is often considered a prerequisite for Quality by Design. Where 22% of respondents noted that development and manufacturing teams communicate closely and often, from the earliest stages or at least from Phase III , the remainder said communication occurred only at pilot or scaleup stage.
Bridging the IT and Manufacturing Divide
Respondents report differing levels of communication between IT and automation groups. Nearly 41% say that communications are ongoing and that the groups discuss projects and issues “as much as possible,” while 24% say communication is increasing. However, roughly one-third report that the two functions are not connecting that frequently or well, with 7% describing the two groups as being “on different wavelengths.”
Respondents this year viewed both alarm management and simulation as far more important than what we’d seen in previous surveys. Nearly 42% said their organizations were applying simulation, mainly to process optimization but also to facility design and construction and building management and operations. 32% said they were using model predictive control. Alarm management began to surface as a priority in last year’s survey.
That trend continued this year, with 67% of respondents noting that they had or planned to develop an alarm management strategy at their facilities. Only 6% reported not having any plans for alarm management. Wireless technology is also being used at more pharmaceutical manufacturing operations, results suggest. Roughly 35% are using wireless for warehouse and some process applications, while 12% are using it for process applications. Another 27% plan to use it for either or both warehouse and process applications, while the remainder say they have no plans to use wireless.