It is within the lab where various phases of drug development occur as new drugs and therapies are discovered and developed through extensive research. Labs are then used to provide quality control monitoring as promising drugs move through process development on a pilot scale, followed by non-GMP and then GMP manufacturing.
During the lab scale research phase, a significant amount of analytical development takes place. Once a promising new drug has progressed through development and shows efficacy, lab-scale process development begins. The next step is process development on a pilot scale, where actual manufacturing processes can be established, along with the establishment of standard operating procedures (SOPs).
In each step, the transfer of processes and procedures must be well aligned with the original lab protocols. Consistency of analytical measurements and technology helps ensure this transitional process progresses from the initial lab activities through to full scale production as planned.
This article will examine how process and analytical instruments are used to facilitate the progression of drugs from the lab to full scale production, primarily by replacing lab-based testing with online monitoring.
The role of lab testing
Throughout the production processes for drugs and therapies, labs serve the critical role of quality control, releasing product in each stage of the process to meet guidelines set by the U.S. Food and Drug Administration (FDA). Without lab sign-off, drugs cannot pass to the next phase of production. These signoffs require extensive lab analysis and process verification.
Pharma processes rely on pH, conductivity, dissolved oxygen, optical density, ultraviolet (UV) absorbance and other measurements. When these measurements are made in the lab via testing, issues can arise. Inconsistency can occur due to sampling techniques, poor sample handling and variations between real-time measurements and lab analysis results. When a sample is extracted from the process, its properties — such as temperature, degree of mixing and other factors — may not match the process media. Further deviation can occur due to improper sample handling or simply inconsistencies among technicians, even when all are following SOPs.
Even if the sample perfectly matches the process media when it finally reaches the lab, there will be a time delay between sample extraction and test results. This delay often makes it impossible to apply closed-loop control to a production process, and it can also make it difficult to implement open-loop adjustments. In both cases, issues arise because the results of changes made to improve upon sample properties are not available for quite some time.
For many properties, the issues with lab testing can be effectively addressed by moving measurements online to create real-time readings.
Lab-to-process digital liquid analysis
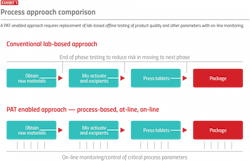
Today, with the application of process analytical technology (PAT), pharma production processes are being streamlined as the analytical tools used in the lab become more available for use in production processes. These improvements are made possible by new instruments and analyzers able to make online measurements for properties formerly requiring lab testing (see Exhibit 1).
One aspect of PAT involves bringing lab measurements out in the process to speed release, improve time to market and cut costs — all while maintaining quality. This approach requires consistency between lab analytical methods and production measurements, which is critical to ensure PAT can be properly and safely implemented.
Implementation of lab-to-process PAT requires transfer of off-line measurements to the process using consistent technology in the lab and in the process.
Transfer of off-line measurement
When discussing the transfer of off-line measurements to the process, common terms are “off-line,” “at-line” and “in-line” measurements.
An example of off-line lab analysis is the use of a benchtop photometer for photometric sample analysis. With this off-line approach, a sample is manually delivered to the lab and analyzed using a benchtop instrument. The sample may or may not require preparation prior to analysis. This type of off-line testing can take hours to perform due to sampling time, sample delivery time to the lab and the schedule of activities occurring in the lab.
At-line (or on-line) analysis involves the use of a field-hardened analyzer, such as a gas chromatograph, to conduct an analysis by drawing a sample directly from the process. In this scenario, the analysis time improves from hours to minutes. Analysis time is the sum of time required to draw the sample into the analyzer and inherent analysis delay time. These types of instruments, such as field-hardened chromatographs, still require careful maintenance to maintain performance.
In-line analysis involves the use of an in-line sensor measuring a parameter directly in the process. This is typically a sensor inserted into the process, which sends a signal to a transmitter. The transmitter uses the sensor signal to create a real-time measurement, which is transmitted to the automation system. Examples of this are in-line Raman and tunable diode laser instruments, along with on-line sensors and transmitters using UV, visible or near infrared light technologies.
Consistent technology in the lab and process
With the advent of digital sensor technology for liquid analysis, industrial processes now have a greater opportunity to cross over from the lab to the process and back.
Digital sensor technology uses digital communications between the sensor and the transmitter. This improves measurement performance by removing interference effects commonly found with analog sensors — such as EMI/RFI interferences and ground loops — while also offering additional performance benefits. In addition, digital sensor technology includes sensor-to-cable connections and internal sensor memory to facilitate ease-of-use.
Originally implemented in process sensors, digital sensor technology can now be applied in the lab. For example, critical pH measurements can be made in the process using a sensor identical to the one used in the lab. Alternately, a pH sensor with a protective plastic body can be used to make the same measurement in the lab, maintaining consistency with a more robust design for repeated handling.
As new drugs and therapeutics are developed in the lab, the same sensor technology can be used in the development lab as will be used later in production. And, with smaller, more compact electronic devices to interface with the sensors, the lab can use the same device used by production to verify process measurement points.
Mobile handheld devices can be used in the lab as well as in the process for spot verifications, driving consistency across the process. These mobile handheld devices often employ Bluetooth technology and apps to interface with digital sensors, and measured values can be easily transferred to host automation systems using Bluetooth communications.
With sensors designed for the process as well as the lab, and electronics that can cross over, consistency is provided from lab to process.
Lab-based computer interface and sensor management
In addition to compact mobile handheld devices for sensor interface in the lab, more sophisticated systems are also available. For example, PC-based sensor management software is available to interface with a variety of sensors for benchtop operation in the lab. This type of software will typically provide sample measurement, sensor calibration and sensor lifecycle management.
Sensor management software provides direct interface to digital sensors in the lab. The software auto-detects a sensor and immediately stores actions in the system database associated with the sensor serial number. Sample measurements and sensor calibrations are stored for immediate retrieval from the system, even after the sensor has been disconnected from the system, and reports provide key regulatory documentation.
Some types of sensor management software can be licensed with 21 CFR Part 11 compliance to include data security, user management and a complete audit trail.
Labs serve as product development facilities and as quality checks on production processes. In its role of quality assurance, a lab will be used to make many of the same measurements made in the production process. To ensure consistency between process and lab measurements, it is increasingly important to use measurement technologies that are as identical as possible from lab-to-process.