Why the surge in generics approvals hasn’t lowered drug prices
In the last few years, the FDA has touted its efforts to bring more generic drugs onto the market as a strategy to lower drug prices. During first five years after the Generic Drug User Fee Amendments (GDUFA) was passed to streamline the process of generic approvals (2013-2017), the FDA approved 2,700 new generics — a 16.9 percent bump from the previous five years.
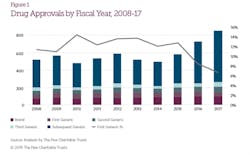
But according to a new report by the Pew Charitable Trusts, the increase was “largely driven” by approvals for the fourth, fifth, sixth or higher version of the drug. And although the first and second versions of generics can greatly reduce the price for patients when they hit the market, the cost benefits decline after the approval of the third version. In 2017, these “subsequent generics” made up 78 percent ANDA approvals.
Despite the fact that generics have saved consumers around $1.7 trillion in the last 10 years (according to the Association for Accessible Medicines), the Pew study also points out that the lack of competition for some drugs is keeping prices high.
For example, there were more than 550 drugs in 2018 without a generic competitor even though there were no patent protections. Generic manufacturers are often hesitant to develop copycats for some of these drugs either because of the complexity involved in production or because they are geared towards rare diseases for a small customer base. And these “sole source” drugs are the most likely to experience price hikes.
Under GDUFA, the FDA has stated its commitment to prioritizing application reviews for first, second and third generics. But Pew ultimately concludes that there are limits to the FDA’s ability to spur vibrant competition in the generics marketplace.
“Policymakers seeking to reduce drug spending may want to consider approaches beyond FDA review to increase competition,” Pew states.
Read the full Pew report.