For years, observers have predicted a “sea change” in the pharmaceutical industry, driven by external economic forces. The result would be more efficient drug manufacturing and R&D, and greater recognition of the strategic importance of drug manufacturing.
The industry has been facing change on a massive scale for the past few years. There is a dwindling pipeline of new drug candidates, and the “patent cliff.” Over the next five years, $92 billion worth of name-brand drugs will come off patent, and, research firm IMS predicts, generic drugs will account for 85% of the U.S. market.
Generic manufacturers, meanwhile, are pressured by high demand but low prices for many of their products, as well as insufficient manufacturing capacity, at a time when compliance and quality problems have driven several suppliers out of the market.
Price controls are being enforced throughout Europe, while, in the U.S., changes in the healthcare system are expected to reduce profitability and drive increased demand for lower cost products. We asked a number of industry analysts whether all these factors had pushed the drug industry to its tipping point. The consensus is a resounding “yes.”
“At the start of this new decade, pharma simultaneously faces the pressures of an ‘innovation gap’ resulting from lack of R&D productivity within large enterprises, reimbursement pressure leading to reduced prices, [the need to] supply a larger market demanding safer products, global competition coupled with global market opportunities, and poor consumer perception,” says Matthew Hudes, national managing principal for biotech, life sciences and healthcare with Deloitte Consulting (San Francisco).
Late to the Party
But, industrial transformation takes time, says Suraj Mathew, partner with the consulting firm, Tefen, Inc. (New York, N.Y.), “If you look at electronics [now at a nine-sigma level], and where it was back in the 1970s, you see that the process takes decades, and the pharma industry was relatively late in adopting operational excellence.”
Most Big Pharma manufacturers didn’t begin their Six Sigma or Lean programs in earnest until around 2000. Before that time, pharma’s efforts to apply these tools were tactical and based on the need to solve a specific problem, says Thomas Friedli, professor at the University of St. Gallen in Switzerland, who has been analyzing pharma’s OpEx practices for the past several years. Today, Friedli says, more pharmaceutical companies are at a more advanced stage in their OpEx evolution, and smaller and mid-sized firms, and even some contract manufacturers, are also embracing the concepts.
“The recent movement of pharma companies to OpEx is similar to what heavier manufacturing companies did in the 80s and 90s,” says Allan Krul, principal for Strategy and Operations with Deloitte. “Added cost and competitive pressure from outside the U.S. forced many manufacturing companies to find new ways to reduce cost, improve operations and product development, reduce time to market and improve overall quality.”
Today, many drug manufacturers’ processes are at 2.5 to 3 Sigma, Krul says. “Over the past 10 years, much of the emphasis has been directed at pipeline development, with a primary focus on speed and cost. As a result, not much improvement has been made to the overall process quality, which has had a negative impact on regulatory compliance,” he says. Given some of the recent regulatory challenges that many companies have been facing, a majority are stepping up their efforts to improve process quality by implementing Design for Six Sigma, Quality by Design (QbD) and other advanced Lean Six Sigma Methods.
However, Tefen’s Mathew acknowledges that, given increased competition and other pressures, OpEx, per se, can be a tough management sell for some pharma companies right now.
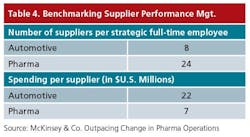
Based on McKinsey & Co.’s pharmaceutical operations benchmarking study, the industry’s current “Right-First-Time” is 95% on average, a figure that has increased, but not much, over the past few years, says Ulf Schrader, McKinsey principal based in Europe.
Roughly half the sites that the analysts studied are still focusing on reducing slack in their systems, which translates into significant efficiency gains. Only the other half has moved beyond this point, and is focusing on in-process checks and control, stabilization, and standardization, he adds.
The Impact of Mergers
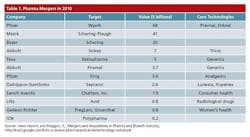
Mergers and acquisitions can delay OpEx efforts, and make them more challenging. According to MedTrak analysts, the pace of pharma and biotech mergers and acquisitions (Table 1) increased by a record 20% last year, and projections call for more activity this year.
Through the Back Door
In other cases, pharma companies come to OpEx through a back door. Jason Kamm, chief consultant with Tunnell Consulting (King of Prussia, Pa.). says that one of his pharma clients has enlisted help because it is unable to meet demand for a key drug. “It’s a nice problem to have,” says Kamm, and a story he is hearing more frequently.
The key to success, Kamm says, is ensuring that quality is part of overall efficiency improvement. This can easily be done through targets such as “yield improvement,” which encompass both efficiency and quality concepts.
“While some have viewed a trade-off between efficiency and quality, more enlightened companies tend to see the synergy in optimizing both,” says Deloitte’s Hudes.
The industry’s biggest challenge is applying OpEx to the D in R&D, an area where Lean and Six Sigma can yield high dividends. Last month, in an interview with the Wall Street Journal, Thomas Lonngren, the outgoing head of the European Medicines Agency, said that pharma wasted over $60 billion each year in its R&D.
Quality by Design is really what other industries have been doing for several years, says Tefen’s Mathew. Work both before and after the experimentation stage can be streamlined, he says, to improve results.
“Clearly, this industry has not placed as much emphasis on process excellence as it has on innovation,” says Hudes. OpEx has often been placed on the “back burner” relative to pipeline enhancement, he says, but the goals are not mutually exclusive.
President Obama’s initiatives will likely have a mixed impact on the industry, experts say. If it passes, one proposed industrial tax credit, designed for manufacturing, might not have a great impact on name-brand drug manufacturers, because it assumes that the companies involved carry high levels of debt. Most pharma companies are still cash-rich. However, the bill could help some generics manufacturers.
Health care reform will put downward pressure on product prices, while pushing increased manufacturing efficiency. Deloitte’s Hudes expects it to exert a positive force for change within the industry.
Even though the drug industry is under pressure, IMS predicts 5% to 7% overall market growth this year. Pharma is also three to five times more profitable than electronics and automotive, says Gonce. “We do see some dramatic price erosions, for instance through the tender process of mutual insurance funds in Germany,” says his colleague, Schrader, “but there remain many areas of the market that are hardly affected. In pharma the quality imperative, rather than cost pressure is likely to be the key accelerator for Sigma level,” he says.
Capital spending in the industry has still been relatively strong for the past two years, compared with other U.S. manufacturing sectors. According to Annette Krueger, VP of research for biopharma and pharmaceutical services at Industrial Info (Sugarland, Texas), spending on new facilities and retrofits increased from $13 billion in 2009 to $15 billion last year, as smaller companies snapped up some of the facilities shed by larger companies.
All the change going on in the drug industry challenges pharma to make OpEx and culture change a higher priority. Every one of the challenges now facing the industry is also an opportunity, says Deloitte’s Hudes.
References
1. Maggon, K. Mergers and Acquisitions in Pharma and Biotech Industry. http://knol.google.com/k/m-a-review-pharmaceutical-biotechnology-industry#
2. Dey, E., Pharma’s woes not over, more restructuring seen. Reuters, November 28, 2010. http://mobile.reuters.com/article/healthNews/idUSTRE6AR1NJ20101128
3. Losch, M., and Schrader, U. Outpacing Change in Pharma Operations, McKinsey & Co., December, 2009