Continuous Chromatography as a Downstream Bioprocess Tool
April 23, 2013
10 min read
When it comes to bioprocess, a number of techniques have been introduced to speed up and improve the yields from the fermentation and synthesis steps. A recent article by Eric S. Langer, “Breaking the Biotech Bottleneck,” (January 2013, Pharmaceutical Manufacturing, pg. 26) discussed the advances in “upstream” bioprocessing that have been made. In fact, when an article about PAT and QbD in the Biotech field is published, it is almost completely dedicated to either increasing yields or speeding up the fermentation process (e.g., in-line analyzers versus traditional lab methods). These approaches are taken to assure a better product, a larger yield, and fewer by-products (due to extra time sitting in a fermentor, waiting for lab results). In other words, they are all about increasing the velocity of up-front bioprocesses.
These increases in yield and faster harvesting times have resulted in more and more product being sent “downstream” to the purification steps in less time. Sadly, the purification technologies downstream have not been able to keep pace with these upstream processing improvements. The result? More “bottlenecks” in the production and delivery of product. Such constraints have the potential to choke off supply and generate severe shortages that ultimately, may keep products off pharmacy shelves. A number of potential solutions were mentioned by respondents to a BioPlan Associates’ survey of 302 global biopharmaceutical manufacturers1 including updating antiquated equipment, fixing supply chain problems, changing older methods used on newer products, etc. However, only ~9% suggested using moving bed LC (a reference to continuous chromatography) as a solution to their downstream problems.
The technique of Liquid Chromatography (LC) has been around for well over a century. The relatively brief history of chromatographic technology starts just after the turn of the 20th century:2
- 1903, M.S. Twsett, known as the discoverer of LC, coined the term “Chromatography” (writing with colors in Greek), for separation of chlorophyll substances
- 1930, Lederer & Kuhn performed a separation of carotin & zeaxanthin
- 1940s, Gas Chromatography (GC) was introduced
- 1950s Thin Layer Chromatography (TLC) & Size Exclusion Chromatography (SEC) were developed
- 1960s, HPLC was introduced, but developed quickly
- 1970s, Reverse Phase (RP) HPLC columns became commercially available
- 1980s, HPLC was developed for process scale separations, (Supercritical Fluid Chromatography) SFC introduced
- 1990s, introduction of process scale enantio-separation via HPLC; Simulated Moving Bed (SMB) developed
- 2000 to present, Capillary Electro-Chromatography (CEC) introduced; advanced SMB operations seen
As far back as the 1950s, Counter-Current (CC) systems were in use as “downstream” processing tools, if only for pilot batches. The CC principle is based on two immiscible liquids in equilibrium in multiple tubes. The sample is added at one end and the tubes shaken. After a rest period (solvents fully separate), the chosen phase (either top or bottom) is transferred to a second tube and the first tube is refreshed with fresh solvent (of the level moved). The apparatus is shaken and the process continued. One positive aspect of CC is that a model may easily be made using a test tube rack and a syringe. The end-product can then tested by dipping a small (sterile) filter paper into the solution, placing four dilutions on an inoculated Petri dish, heating at 37ºC overnight, and reading the “kill-radius” to determine product purity.
In 1965, staff at Merck, myself included, used a 102-tube counter-current unit (the size of a small car) to purify fermentation broths (of development or pilot plant size). The ends of the tubes were highly polished and cleaned with a chamois as they needed to be ultra-clean. Despite precautions, the tubes tended to leak and there was often carry-over of the wrong phase. Needless to say, the process, while effective, was eagerly replaced by preparative HPLC soon after.
Today, the most popular method of downstream purification is preparative LC, available since the 1970s. While effective, prep LC can be slow and have other drawbacks. The typical procedure involves placing a large, concentrated “slug” of biomaterials on a large column (perhaps a meter in diameter and several meters long), packed with an ion exchange resin, silica gel, or some other packing. An eluting solvent is pumped through the column, often at several liters a minute, in an attempt to separate the wanted drug (protein, enzyme) from nutrients, waste, side products, etc. Unfortunately, the majority of methods cause overloading of the column. This causes tailing and mixed peaks to elute; these cuts are then recycled through the column until “purity” is declared.
This repeated on-column time that the product spends cause a number of potential problems:
1. Wasted time—Depending on the product and amount of materials needed to be eliminated, the purification can take nearly as long as the synthetic step.
2. Multiple losses—Multiple passes through a column mean multiple losses. A percentage of the product is lost (just as in a recrystallization of a small molecule drug) each time through the column.
3. Breakdown of active ingredients— There is a danger of denaturation, causing potential loss of activity. (Silica columns are “passified,” so as not to react with the product, for instance, by denaturing a protein structure, but can change with use.)
Moving Bed Method
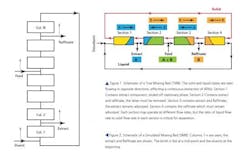
A more efficient method is a “Moving Bed” (either True or Simulated) chromatography system, wherein the materials to be separated or cleaned up are continuously injected into one end of the system and purified material is collected at the other (and unwanted materials continuously eliminated). While this sounds simple in principle, the engineering can be complex and needs to be quite precise. The skills of chromatographers and engineers need to be melded.
While the first True Moving Bed (TMB) systems were designed to separate two materials into two continuous streams of “pure” eluents, the concept may be also used to separate one API (e.g., a protein) from its fermentation broth. However, a “True” moving bed system is not practical, since the solid adsorbent needs to be pumped in opposition to the flowing liquid phase (See Figure 1); and it’s an engineering nightmare. It is far more practical to pump the liquid phase through a series of fixed bed columns (See Figure 2). To simulate a counter-current effect, the feed, eluents, extract and Raffinate (the left-over materials) lines are all moved from column-to-column at fixed time periods.2 It turns out that even a small number of “zones” or columns in a Simulated Moving Bed (SMB) can give results approaching an ideal TMB system.
Continuous chromatography links several columns (as many as eight) in a looped, end-to-end configuration. This “chain” contains (in addition to a solvent inlet) inlets for the raw product, an outlet for the target product, and one for the discarded materials (Raffinate). Defining the process as “continuous” means the feed, extract and Raffinate ports cycle their effluent from column to column, but lagging behind the solvent flow.
The overall effect is as if the stationary phase were a moving component, just in the opposite direction of the solvent flow. Based on preliminary method development, the stationary phase, mobile phase, flow rate and amount to be loaded on the column are determined. When properly designed, the process appears to be dividing the product stream into two continuous streams; one flowing downstream and one flowing upstream.3
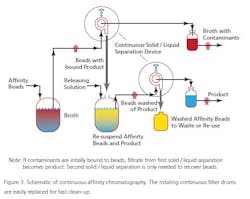
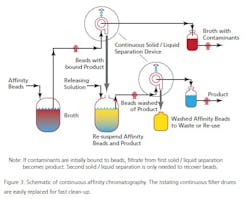
At the moment there are a limited number of companies offering this technique as a practical service, but as the technique proves useful more will, no doubt, offer such engineering support. The technique, while not new, has been updated for use in the 21st century applications of PAT and QbD. One example is Novasep’s “Varicol” set-up (See Figure 3). During a separation, the solvent is recovered from evaporators and dryers and the pure dry compounds recovered. The recycled solvent is reused both as eluent of the CC system and to dissolve the dry feed mix. A small amount of fresh solvent is added to the recycled one, adjusting the eluent composition. The organic solvent is recycled of 99+%; the solvent consumed is as low as 130 mL per kg of purified product has been reported (by UCB Pharma).
Since unlike SFC and HPLC, multicolumn continuous chromatography is a continuous process, it is typically as much as 10 times more productive than a batch chromatography process.
MCC processes, such as SMB and the Varicol, are designed for the separation of binary mixtures and thus, for instance, are perfectly adapted to the resolution of racemates and diastereomeric mixtures. In multicolumn continuous chromatography processes, one long column is replaced by a loop of 4 to 6 shorter columns, optimizing the usage of the stationary phase. Only pure fractions are collected, leaving mixed fractions to re-circulate through the columns.
Recent Literature Offers Examples
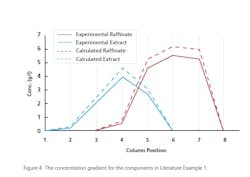
Example 1: An enantio-separation of a Drug Candidate via SMB which was a Partial agonist for muscarinic receptors. The adsorbtion isotherm was determined by measuring enantiomer retention time and the amount of racemic compound loaded on column. The throughput is 125 g rac./day using 8 columns in a 2/2/2/2 configuration. Each column is 26x105 mm packed with ChiralPak AD (20 μm). The Raffinate is 99.5% eliminated with the Extract extracted at 97.8%. The total recovery is greater than 98%. Figure 4 shows the levels of materials and their locations on the columns during a separation.4
Example 2: The SMB enantio-separation of α-ionone and α-damascone was slow and expensive with conventional LC. The Racemate is produced industrially from acetone and citral. It is enanioselecive via racemic epoxide precursor; the threshold concentraions for (R)‐α‐ionone and (S)‐α‐ionone are 0.5‐5.0 μg/kg and 20‐40 μg/kg, respectively. (The Racemate was produced industrially from allyl Grignard and α‐cyclogeranic acid methyl ester.) There is also an enanioselecive route via stoichiometric chiral amino alcohol resulting in (R)‐α‐damascone 100 μg/kg, (S)‐α‐damascone 1.5 μg/kg.
Both enantio-selective synthesis routes had issues with scale‐up, but SMB provided a convenient alternate for resolution with α‐ionone α = 1.6 and α‐damascone α = 1.3 (5).
The method developed used eight 12.5 x 1.0cm columns containing 10µm Nucleodex-β-PM® (Macherey-Nagel); the eluents was 70:30 MeOH:H2O @ 100C. The void fraction was determined experimentally by MeOh injection into each SMB column. The Henry constants (a.k.a. adsorption equilibrium constant, it is expressed as a ratio of the analyte excess adsorption to its equilibrium concentration at the very low concentration level; Analyte retention in HPLC is proportional to its adsorption coefficient) were determined experimentally by the retention time of each enatiomer.
For α-ionone, optimized parameters yield 0.72 g rac./day with 99.0% purity of the S and 99.2% purity of the R enantiomer. For α-damascone, the optimized yield was 0.22g rac./day, with 99.9% and 98.8% purities for the S and R, respectively.
If any purification process can be successfully adapted to SMB by the biotech industry, shortages of important, high-demand biomedicines may become a thing of the past. While a small number of companies now employ the approach, there’s been a spate of research papers published on the topic. Figure 7 shows how the number of papers on the subject has exploded over the last two decades. Now, we wait to see the logjam disappear.
Published in the April 2013 issue of Pharmaceutical Manufacturing magazine
References
1. “Breaking the Biotech Bottleneck,” E.S. Langer, Pharm. Manuf., 12 (1), January 2013, pp. 26-8
2. R. Ditz, Introduction tp Preparative Chromatography, Henner Schmidt-Traub; Wiley-VCH: Weinheim, 2005
3. “Complete Design of a Simulated Moving Bed”, F. Charton and R-M. Nicoud, J. Chrom A, 702 (1995) pp. 97-112
4. Guest, D. W. J. Chromatogr. A 760, (1997) pp.159-162,
5. Morbidelli et al. Flavour Fragr. J. 17, (2002) pp.195-202
6. Mazzotti, et al., J. Chromatogr A 1216, (2009) pp. 709-738
About the Author
Emil Ciurczak
Contributing Editor
Sign up for our eNewsletters
Get the latest news and updates