How to Optimize Continuous OSD Manufacturing
The pharmaceutical industry has matured quickly over the last few years, responding to regulatory changes and significant technology advancements, while undergoing a period of unprecedented mergers and acquisitions and global economic uncertainty. These factors have begun to transform the industry into a leaner model with a higher degree of focus on operational costs and risks.
Traditional manufacturing industries have undergone similar disruptive lifecycles and have adopted production methods to optimize profitability and maintain a competitive edge. A key lesson learned from other manufacturing industries is that facility operations and profit-ability can be favorably impacted by converting manufacturing from batch production to continuous operations. The transition from batch to continuous operations generates multiple cost savings and operational efficiencies.
Within the pharmaceutical industry, secondary manufacturing of oral solid dosage (OSD) forms has traditionally been a batch-oriented operation with some semi-continuous unit operations. This production method has been reinforced by the lack of enabling technology and by regulatory standards. In recent years, however, equipment manufacturers have had increasing access to advanced technology to enable the transition to continuous operations, and new continuous processing equipment now addresses most unit operations involved in OSD manufacturing. In concert, regulatory agencies have adopted batch and lot definitions that facilitate the inclusion of continuous processing.
This article intends to review the advances in continuous oral solid dosage manufacturing from an engineering design firm or integrator’s perspective.
Today’s Batch Oral Solid Dosage
The production unit operations associated with OSD manufacturing have historically been designed for batch processing. A batch, defined under the Code of Federal Regulations (21CFR210.3) as a specific quantity of a drug or other material that is intended to have uniform character and quality, within specified limits, is produced according to a single manufacturing order during the same cycle of manufacture. Traditionally, batch processing is characterized by a step-by-step series of operations or workstations that combine, in series, to complete all processing requirements for a particular product. Batch processing provides a natural means of product quality control as the product in production is confined to the finite batch quantity and after each step or series of steps, the product can be evaluated (tested) to assure it conforms to specified uniformity and quality parameters.
In the OSD industry, the most common manufacturing steps may be easily identified:
• Weighing and dispensing
• Granulation (wet, dry or direct)
• Particle sizing and blending
• Tablet compression
• Tablet coating
• Packaging
The integration of the above steps into a batch process on a commercial scale results in large facilities primarily due to the need to have space for staging, material handling, corridors and multiple processing rooms. Low utilization of equipment is characteristic of batch processes, mainly due to the differences in equipment residence time, cleaning and equipment setup. The differences in processing time often require the addition of work in process (WIP) storage.
At a commercial scale with large batch sizes, material transfer becomes a significant operational consideration as well. Batch sizes in the hundreds of kilograms are not unusual and can make processing a labor-intensive operation with challenges to the safe and efficient transfer or transport of the batch contents among the different workstations. Due to inherent characteristics, large batch processes generally require a greater initial capital investment, longer processing times and higher operating costs.Continuous Processing
As a manufacturing technique, continuous processing supports product production for extended periods of time without interruption. This approach permits 24-hour production cycles with minimal or no interruption. The extended utilization of processing equipment often results in a production line comprised of smaller equipment which can produce more product compared to similar batch processes. Concerns associated with material handling, WIP storage and production floor space commonly found on a batch process are largely resolved by continuous operations, which minimize all three requirements. This in itself has a potential to reduce the costs associated with the product manufacture, including the initial capital investment. Regulatory agencies opened the door to continuous processing by defining a batch to a production timeframe of no more than 24 hours.
Experience has shown that continuous processing presents new challenges, primarily associated with regulatory requirements for material tracking and quality control. These challenges include:
• Multiple lot tracking of ingredients;
• Balanced throughput among unit operations, and
• Online process control.
A true continuous process behaves as a plug flow process and is based on first-in, first-out without back mixing or accumulation. These are all requirements to maintain homogeneity.
In solids processing, maintaining the concept of plug flow and homogeneity requires careful consideration due to inherent characteristics like segregation and agglomeration. Here, the integrator must scrutinize the equipment offering and product characteristics carefully to properly match them to the particular continuous process. This is especially important because continuous processing has the potential to produce very large product losses. Therefore, the proper match between equipment and product becomes highly relevant.
Engineering Firm As Process Integrator
When any engineering firm is engaged to develop a pilot or commercial-scale continuous process operation, it becomes the process integrator. Most all aspects of the project, from facility design to equipment procurement are handled by the engineering group. With most projects, the owner provides basic process and production requirements and the engineering firm provides the services to, not only integrate the process line being developed, but integrate it with the ancillary operations of the entire facility. As part of these services, the integrator often must challenge the conventional wisdom of what may seem to be a proven commercial solution and create the best solution to meet specific production requirements and continuous process goals.
Integrators take on the responsibility for capital and operational cost optimization. This becomes more evident where opportunities for continuous processing are a feasible, rational alternative to batch processing. Determining the efficacy of one production method versus another requires careful analysis and a deterministic approach if one is to reach a processing decision that will have a profound impact over the lifecycle of the product. The integrators’ industry perspective becomes a valuable asset in this process since the experience gained from successfully executing a variety of projects for multiple clients does provide a broader baseline to assist in the decision making.
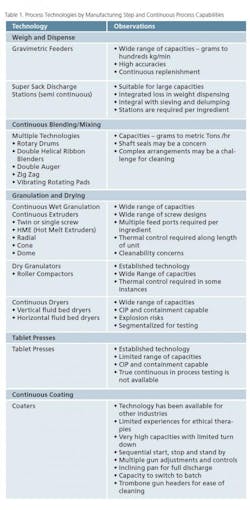
Process equipment manufacturers have made significant advances towards engineering complete product lines that support continuous processing of pharmaceutical solids. The effort continues and the contributions from innovative manufacturers have been outstanding and enabling to the industry. Other technologies are rational adaptations of equipment currently used in today’s batch or semi-continuous processes. Table 1 offers a short list of processing technologies (by manufacturing step) and observations as to the capabilities associated/required for continuous processing.
Heuristics – Batch vs. Continuous
Advances to continuous oral solid dosage processing will prompt pharmaceutical manufacturers to quickly evaluate and determine the application of one production technique over another. In classical chemical plant design, the first choice is to plan for a continuous process. In secondary pharmaceutical operations this is not the case. Over time, integrators gain experience and tend to develop a baseline on certain “rules of thumb” which ultimately support better decision making and allow them to more accurately rule in favor of one technique over another.
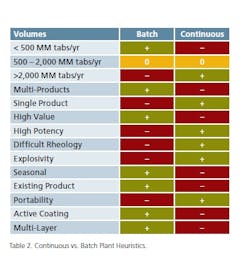
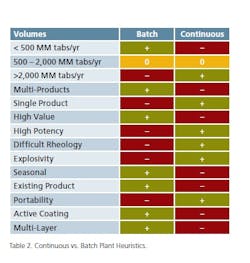
A primary discriminating parameter is associated with production volume. Production of large volumes of solid material in a pharmaceutical environment is a challenge for batch and strongly points towards adapting a continuous processing solution. As noted, material handling alone is a major undertaking that is significantly mitigated with continuous processing. Lower production volumes are common and justified in multi-purpose facilities. Production in the order of 200 MM tablets/yr is common for batch-based multiproduct facilities. In contrast, dedicated facilities producing volumes in excess of 2,000 MM tablets/yr are a prime candidate for conversion to continuous processing.
The manufacture of multiple products in the same production facility, and in particular, when processing equipment is shared, introduces additional production/quality requirements associated with cleaning, testing and change-over of parts. These multi-product production requirements introduce interruptions as they create extended pre/post-production processes that reduce the efficiency (and advantages) of a continuous process and impact the available production capacity. In addition, with certain operations such as blending, drying, granulation and coating, residence times will vary from product to product. Matching these processing requirements with available equipment is not always possible within the turndown parameters on individual components for a continuous processing line. In the evaluation between continuation and batch processes, it must be considered that batch processes are more suitable to accommodate these differences.
PAT Is A Must
Critically, continuous processing requires a comprehensive structure for process control. The implementation of process analytical technologies (PAT) becomes a major requirement for continuous operations. Batch operations allow the test and release of intermediate production and minimize losses. Lack of a rigorous PAT structure for continuous processing may result in potential losses of large amounts of product and introduce concerns regarding quality control. For high value products, the potential loss of significant amounts represents a major financial concern.
Material handling, as mentioned, is another major aspect of batch processing. Product characteristics including potency, rheology and explosivity often make material handling procedures significantly more difficult than others. The engineering team integrating a process devotes a significant amount of time solving these problems. Opening process equipment for cleaning, loading and unloading increases these risks. In comparison of batch vs. continuous, batch processing requires more direct access to the equipment more frequently than for continuous processing. These factors can make continuous processing a better choice for products with these characteristics.
Seasonal products create large swings in production demand. So in the “off” season, facility utilization is likely very low and that drives the need for additional products to fill the void created by seasonal products. This generally makes seasonal products more suitable for multi-product, batch-based facilities and offer little rationale to implement a continuous process to support production.
Possibly the biggest challenge to determining the application of continuous or batch processing is when a new facility is planned for an existing product. Established products often include specific processing steps that may not be suitable for continuous processing. Examples of these processes include: manual screening, a particular order of the introduction of ingredients, pre blending an aliquot and other complexities associated with formulation characteristics. These processes often require the development of “bespoke” continuous methods to mimic manual operations. As a result of existing processing methods, the product is probably better suited to batch processing. New production facilities that are designed for continuous processing should be developed to take advantage of the improved commercial performance that is inherent in continuous production. In parallel or ahead of this commitment, products need to be developed with the capability of employing continuous processing.
Table 2 captures the experience on continuous and batch oral solid dosage processing. This is not to be considered as a definitive set of rules, but as a starting base on the long road to a mature processing technology.
Integrators’ Perspective
Engineering firms that act as an integrator of owners’ requirements, production constraints, business and capital objectives, find a stochastic view of the implementation of a continuous process is an effective approach. Such firms, including Fluor, have a distinct opportunity to look both at the micro and macro of the Integration process. Following are some general observations Fluor has collected on the state of development of continuous oral solid dosage:
• Integration of PAT at critical unit operations is critical to maintain quality and avoid product loss.
• Supplying a continuous process with a large volume of excipients requires more careful logistics planning and control at the warehouse level.
• Use of bulk bags requires modifications to standard warehouse design and planning
• The overall Continuous Process response to individual unit operations hysteresis is a concern since the available equipment may not deliver proper response and turndown ratios.
• To support quick change over, it is necessary to determine the application of CIP or dedicated change parts and components.
• Disparity in OSD Continuous Processing can often be traced to Equipment throughput and turndown.
• Equipment processing duration during a production run varies from stem to step. The longest duration limits the overall line capacity.
• Pay attention to Transient operations and response to changes.
A Viable OSD Approach
There is overwhelming evidence that continuous processing is a viable approach to manufacturing OSD therapies. There are a limited number of successful projects utilizing continuous operations at various levels from a few unit operations to the entire process. However attractive it may appear, the broad spectrum of products and production requirements makes it clear that continuous processing is not the solution to all manufacturing processes. At Fluor we have observed that more often than not, implementation of a continuous processing line revolves around mimicking a batch process, which may not be the most efficient means of continuous production. Perhaps the largest challenge is the development of standard guidelines for continuous OSD equipment manufacturers to consider overall line requirements.
Continuous oral solid dosage has the potential to improve business opportunities to drug manufacturers, and at the same time, sustain broad affordable therapies for the patients. This is done through labor cost reduction, reduction of manufacturing space, energy savings, reduced time-to-market and real time quality control.
Published in the April 2013 issue of Pharmaceutical Manufacturing magazine
Acknowledgment:
Zeinab Alwan – Fluor, Marketing, Manufacturing & Life Sciences Timothy McNeil – Fluor, Director of Technology, Manufacturing & Life Sciences