Formatted Active Carbon Filters
Sept. 6, 2013
5 min read
The evolution of charcoal from its earliest uses in ancient Egypt and India for the clarification of curatives and drinking water to its present activated carbon form is characterized by the selection of carbon sources, methods of activation, materials design and complex formations. Concomitantly, uses of activated carbon have expanded to include in vivo medicinal treatments, toxic waste remediation and catalysis to cite a few examples.
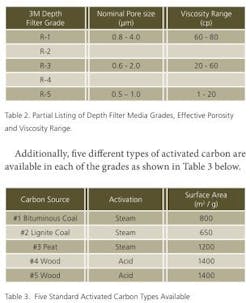
Although water purification utilizes the largest quantity of activated carbon, clarification and purification of pharmaceutical intermediates and products still remain a significant application. Although these latter applications may, by virtue of scale seem less daunting — an application may be unique and used only in a single campaign — they have peculiar requisites: As a general rule, animal sources are undesirable, chemicals involved in activation must be absent or readily removable, and regulatory support material concerning the activated carbon materials should be available. Table 1 lists essential aspects of activated carbons in terms of their sources, activation methods and available formats.
While the characteristics of various grades of activated carbon in terms of porosity and general adsorption capacity are well understood, the phenomena of chemical adsorptions and predictive behaviors are oftentimes enigmatic. This circumstance partially explains the large number of commercially available activated carbon offerings and something that usually necessitates the testing of representative carbon samples with one’s product.
CONSTRUCT AN ISOTHERM
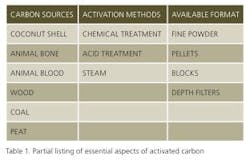
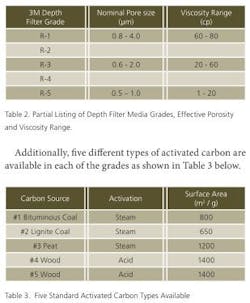
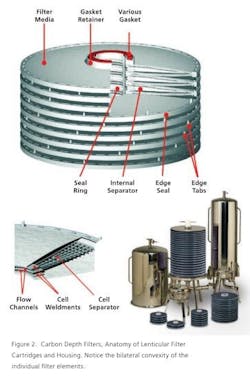
One usual method of evaluating the efficacy of a powder carbon sample with a given product is to construct an isotherm by adding graduated concentrations of a given powdered carbon sample to a product, agitating it for a given time then analyzing it. While not particularly difficult, this process can be tedious and time consuming. Moreover, the realized affect of the carbon may be quantitatively different when small samples are tested in the laboratory and scaled-up processes are actually run in production under quite different fluid management techniques. In contrast to working with powdered activated carbon samples, 3M depth filters impregnated with specific carbon types support both activated carbon selection and production scale-up calculations. For example, Table 2 lists five grades of depth filter media of differing effective porosity, for use with products of differing viscosity. Media with larger porosity are appropriate for viscous samples, while the denser or tighter media are recommended for use with aqueous or solvated samples.
OPTIMIZING CARBON EVALUATION
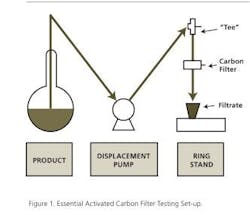
For initial evaluations, small disposable filter capsules of 25 cm2 area, or 13.5 cm2 disks for use in reusable stainless-steel test cells can be used. Using a simple arrangement of filter, holder and a pump for control of sample flow rate, it is possible to optimize both carbon evaluation and operating conditions for scale-up. Figure 1 shows the simple set-up for use in various activated carbon filter media trials. Qualitative analyses of the developing filtrate fractions determine the end point of the testing, and the filtrate pool constitutes the throughput.
Using a known filter area and selecting a product flow rate establishes the flux:
flux = flow/area
generally normalized to L/min/m2.
Given that the filter media area is fixed by the test filter size, the flux will be controlled by the pumped flow rate. The lower the flux, the longer the product residence time within the carbon matrix; and as a general rule, the longer the residence time, the more effective the carbon-product interaction. Once an effective carbon type is selected, generally only one or two flow rate evaluations are required for flux optimization. Following these determinations, it is a simple matter to relate product volume, available processing time, flux and throughput to predict the necessary filter area for a scale-up process. Likewise, existing processes that use loose activated carbon can be easily converted to the depth filter activated carbon format.
It is worth mentioning that the presentation of the activated carbon to the product in the depth filter format is quite intimate and the fixed carbon particles provide consistent interactions with the product. In contrast, the loose carbon powder suffers random packing and is prone to channeling and the selection of preferential pathways.
In addition to simplifying the activated carbon selection and scale-up processes, the lenticular activated carbon filter cartridges obviate the handling of bulk powdery carbon and the associated problems of health and safety risks due to dust, storage and handling as well as post-use clean-up and disposal.
About the Authors
Dennis Battersby is currently the Scientific Applications Support Service specialist (SASS) at 3M Purification in the Life Sciences Process Technology Group. He assists many BioPharma companies with optimization of their purification process. He can be reached at [email protected].
Majid Entezarian (Ph.D.) is the manager of Scientific Applications Support Service (SASS) at 3M Purification. He can be reached at [email protected].
About the Author
Dennis G. Battersby and Majid Entezarian
3M Purification Inc.
Sign up for our eNewsletters
Get the latest news and updates