Reader survey results: Grading the FDA
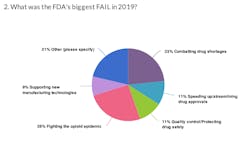
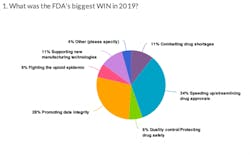
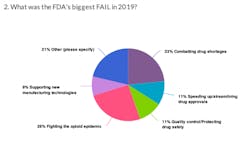
At the end of last year, we asked readers to give us their take on how the FDA did in 2019. Specifically we asked readers to name the agency's biggest win and its biggest fail.
The survey corresponded with the release of our December cover story, "The recall effect" — an in-depth look at how the blood pressure recalls have impacted the industry and how the FDA is attempting to improve quality control throughout the global supply chain.
Here's what you had to say on the FDA's performance in 2019:
Reader comment: "Moving closer to permitting interchanging of biosimilars with the guidance, 'Clinical Immunogenicity Considerations for Biosimilar and Interchangeable Insulin Products.'"
Reader comments: "Not encouraging and promoting drug manufacturing and development in the U.S. to a greater extent. U.S. drug manufacturing and development continues to move off shore. At a time of crisis or emergency in those countries, the U.S. will encounter a huge drug crisis."
"Failing to deal with all the public outcry over price increases such as EpiPen."
"The FDA needs to be less flexible on their inspections."
Thanks to everyone who participated in the survey!