What’s Old is New Again
Environmental conditions are monitored across production and storage facilities, and superimposed on building layout diagrams.
In the pharmaceutical industry, the chemical integrity of compounds relies on the strict control of hundreds of variables from air humidity and freezer temperatures to mixing times. Compounds like Active Pharmaceutical Ingredients (APIs) may be used in anything from prescription drugs to cough drops.APIs and other high-purity substances rely on strict environmental control for production. These controlled substances and pharmaceutical compounds must be perfectly consistent across each batch, so records of every aspect of the process must be maintained — from processing to transport to delivery.Like its global pears, Merck & Cie, located in Schaffhausen, Switzerland, must manage the operational intricacies of GMP-based compliance to be successful. Merck & Cie is a subsidiary of Merck KGaA in Darmstadt, Germany, understood as the world’s oldest pharmaceutical and chemical company with roots dating back to 1668.
FDA AND EU REGULATIONS POSE CHALLENGES
Merck & Cie operates within the strict guidelines of Food and Drug Administration (FDA) 21 CFR Part 11 and European Union (EU) Annex 11 for the development, production, transport and delivery of active pharmaceutical ingredients and high-purity substances.
The company enlisted the aid of engineering firm Retel Neuhausen AG to develop a Good Manufacturing Practices (GMP) monitoring system, a requirement of the FDA and EU for the production of pharmaceutical ingredients and products. The system needed to meet the needs of a large international life sciences company, deliver reliable and accurate data, and adhere to rigorous international guidelines.
One of the biggest challenges in creating any GMP-compliant process control is following the long list of federal and international regulations for record keeping and control. FDA 21 CFR Part 11 details a set of guidelines for data management and the use of Electronic Signatures (eSignatures). The EU Annex 11 standards address computer-based systems, which must also adhere to Good Automated Manufacturing Practice (GAMP) guidelines.
Under these guidelines, not only must process data be recorded, but also environmental conditions of rooms, storage and production facilities. In addition, security is mandated to keep records of user access to equipment and materials. This requires traceability features such as individual user access with protected user names and passwords, and the verification of eSignatures.
The main objective of this project was to create a single GMP Supervisory Control and Data Acquisition (SCADA) system that would strictly follow FDA and EU requirements. Diverse requirements and technologies would have to be brought together into one overall system, and then integrated into a reliable system that could operate across different departments. This required creation of a central GMP SCADA system that would combine monitoring of the facility, locks, freezers/refrigerators and product lines.
MEETING REQUIREMENTS, SAVING MONEY
Retel Neuhausen developed a central GMP monitoring system that comprises a SCADA system using InduSoft’s Web Studio installed on an ESXi server, an enterprise-level computer virtual machine. Web Studio was selected over competing products because it demonstrated its ease-of-use and ability to interface with a large number of automation systems at a lower cost. The product’s compliance with FDA and EU regulations was also an important factor.
Data inputs to the SCADA system include a number of measured values representing environmental conditions such as temperature, humidity and air pressure. These measured values are digitized by decentralized data nodes that are connected to the ESXi server via Ethernet.
The decentralized placement of data nodes helped reduce installation costs considerably as it eliminated long, home-run wiring from measurement devices to the server. Instead, each node is now connected to a number of local sensors, and data from these sensors is multiplexed and transmitted to the server.
Using these distributed data nodes, the readings from the sensors are monitored and recorded. Multiple redundant data acquisition links and paths ensure the system will remain operational at all times.
Because of the over 240 drivers native to InduSoft Web Studio, Retel Neuhausen was able to integrate existing external systems such as autoclaves, production lines and reactors with the GMP SCADA system. These drivers allowed for easy integration of various protocols into the SCADA system without the need for custom programming and driver development.
INTUITIVE INTERFACES ENABLE STRINGENT SECURITY
A number of operator interface display screens were developed for the project using a combination of built-in Web Studio graphic elements and custom-designed elements. Web Studio allows users to quickly build custom elements and perform seamless integrations between built-in and custom elements. For example, users can create displays that convey relevant information at a glance, with the functionality to drill down for further details as desired.
Users can access the GMP SCADA system from any office computer, or via web thin clients while away from the plant floor. This local and remote access increases the efficiency of all users, particularly the engineering staff as it allows them to perform analysis off-site.
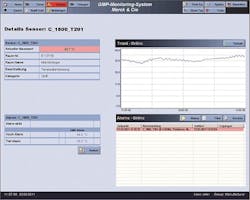
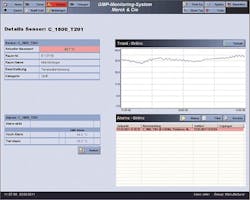
The intuitive user interface means staff only needs a short introduction before effective use of the SCADA system. Various screens show building and floor layouts, providing a quick overview of facility floor plans.
Detailed floor plans of every building show real-time environmental data.
Data is logged and monitored in real time. If values exceed accepted levels, users are informed via an alarm. The first step of the alarm system turns on locally mounted alarm lights. In the second step, an alarm signal is given by the alarm server and transmitted to mobile phones using SMS, and also to land-line phones.
Data is also sent to an online SQL database using InduSoft’s patented database connectivity that delivers exceptional speed and reliability. The data archiving to the SQL database historian reduces the load on the GMP SCADA system, allowing for consistent and reliable server performance. Historian data can be represented graphically in historical trends for easy analysis.
The GMP SCADA system is integrated into a GMP network specially built for the project. The GMP network is connected with the office network through a firewall. The security system ensures that only authorized users can make changes to system parameters.
In addition, the GMP SCADA system uses InduSoft Web Studio to provide tools that designate users as individuals or groups, thus restricting access to different aspects of the system. This helps ensure that eSignatures are verified, and that all user actions are recorded for traceability purposes. As a further enhancement, InduSoft Web Studio can be connected to Microsoft Active Directory using the LDAP protocol to provide advanced SCADA security capabilities.
Historical trending allows users to go back weeks or even months and compare past and current data.
CUSTOM MODULES MEET CLIENT CONDITIONSCustom modules were developed to enable evaluation by the Statistics Report, which displays trends over the course of several weeks or months. These trends offer valuable features such as the number of times value thresholds exceed minimum, maximum and average values.Production processes and sterilization cycles can be evaluated automatically with the Reference Book module. On the basis of a defined setpoint value, each process is subsequently evaluated based on the template qualification laid out in the Reference Book. An evaluation report is then generated for release along with the finished product.
Representatives of the FDA and EU Annex can easily audit and verify data captured with the GMP SCADA system as all development was done in accordance with GAMP 5 and FDA 21 CFR Part 11 in order to maintain Design Qualification, Installation Qualification and Operational Qualification verification.
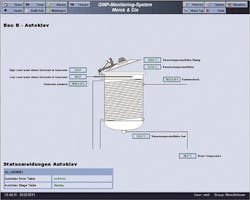
Autoclave chamber and generator pressures as well as lid, chamber and drain temperatures are clearly displayed. An imported graphic provides an unmistakable understanding of the process.
Clear limits for values and carefully planned guidelines give operators the precise information needed for quality assurance. Operator interface screens use standard colors and graphics to make analysis easy, from historical data and trends to everyday operations. Off-site facility monitoring reduces response time, and the ability to access to the system via existing hardware such as PCs and smart phones saves time and money.Retel Neuhausen led the project from planning to completion. By purchasing a turnkey solution, Merck & Cie vendor and contractor interfaces were reduced to a minimum, supporting the successful delivery of the SCADA system that now spans the entire facility. Ultimately, the system not only meets tough international requirements, but was also designed to manage future growth and changes. When new technology is introduced or when production processes change, the system will allow for seamless connection to new hardware and easy access to new data.