Traditional biologic therapies like monoclonal antibodies (mAbs) and recombinant proteins have been positively impacting human health outcomes for decades. As of 2022, more than 100 mAb therapies have been approved by the FDA.
Along with the ongoing success of traditional biotherapies, the biopharmaceutical industry has seen a sea change in workflow types and speeds since the start of the COVID-19 pandemic. New and exciting therapeutic areas have been opened, and researchers, suppliers and manufacturers alike will have to be flexible to meet rapidly growing needs. An urgent demand for new treatments and the introduction of new modalities such as mRNA vaccines and therapies have led to a surge both in speed of development and speed to market. At the same time, supply chain disruptions and material shortages have challenged the industry’s ability to meet these newly shortened timelines.
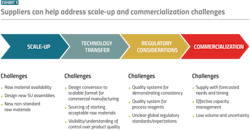
These combined challenges present a heightened need for new efficiencies, which can be developed through collaboration between raw material suppliers and manufacturers. Solutions to problems of scale-up, quality and regulatory considerations and commercialization timelines will need to address cost, throughput and material availability.
Specifically, current challenges can be addressed through the provision of globally sourced, cGMP process materials for in-vitro transcription and capping steps, and the identification of more effective chromatography technologies.
Efficiency challenges
Availability of high-quality raw materials
One of the most important factors in the production of consistent, high-quality pharmaceuticals is the availability of raw materials that are consistent, high-quality and reliably sourced. This challenge is especially relevant when it comes to emerging modalities such as mRNA vaccines and therapies, which demand large quantities of high-quality plasmid DNA (pDNA) to serve as a template for the desired mRNA sequences. Currently, pDNA demand is massively outpacing supply.
Issues in the pDNA supply chain have led to difficulties in scale-up and regulatory hurdles. The first of these issues is the long lead times and high expense of outsourcing the production to contract manufacturing organizations (CMOs). CMOs that can produce high-quality pDNA usually have long waiting lists, which often prioritize larger companies and those with which the relevant CMO has an existing relationship. When these roadblocks are coupled with a high potential for batch failure, it becomes clear why pDNA is the primary bottleneck in the development of not only mRNA vaccines and therapies but in a variety of cell and gene therapies and nucleic acid products.
A similar inefficiency occurs with capping enzymes used in the in vitro transcription phase of mRNA workflows. Capping must occur either during or after transcription to limit the degradation of an mRNA strand. Some capping reagents are expensive and should be used in ideal conditions to avoid material waste. On top of this, a precise ratio of enzymes to plasmids is necessary to ensure that the resulting mRNA is capped and transcription is not limited. This process is sensitive to buffer conditions, time and temperature, so minor mistakes leading to batch failure could drastically increase the expense of this stage of mRNA workflows.
Ways to improve downstream process chromatography of mAbs
Reducing the number of chromatography steps:
If manufacturers can reduce steps by using a selective resin or a resin washing mechanism, this will immediately reduce complexity. For example, downstream purification of mAbs often includes the use of a protein A chromatographic resin for affinity chromatography. Since this step has been shown to provide good purification, typically only one or two standardized polishing steps (such as cationic exchange followed by ion exchange) are needed after protein A.
Utilizing a single-use system:
A single-use system for buffer preparation and media preparation eliminates time-consuming and costly cleaning steps, making these processes available on-demand to improve overall efficiency.
Use a continuous downstream process:
The current standard workflow involves eluting the material from a first column, then storing it in a holding tank before it is prepared and loaded into a second column. This process is not truly continuous because of the holding step. It is possible for some molecules to elute directly from the first column into the second column, without the intermediate storage step in a tank.
Downstream purification
Downstream process chromatography is a late-stage step of many workflows (from traditional biologics to nucleic acid products) but it is considered a major bottleneck in mAb workflows. Since mAbs constitute more than 50% of biologics currently on the market and downstream processing accounts for as much as 80% of the cost of producing mAbs, improving downstream process chromatography could significantly impact the finances and time a manufacturer must dedicate to a product.
Even though chromatography has long been a part of biopharmaceutical workflows, the processes used will need to be significantly refined and improved to yield more efficient and economical outcomes for manufacturers. One inefficiency in chromatographic processes is related to the increased safety features required to account for variations in upstream processes, some of which might result from inconsistencies in raw material supplies.
Chromatographic processes themselves also feature complexities that can give rise to inefficiencies. Different drug substances react differently to chromatographic resins, meaning that desired products and impurities might coelute. This necessitates more chromatographic cycles, potentially with a variety of resin types and methodologies. Improvements to the specificity and dynamic binding capacity of resins, such as the protein A chromatographic resins used in affinity chromatography, can offer solutions to downstream bottlenecks in mAb workflows.
Solutions
Raw material suppliers are uniquely positioned within the biopharma industry to alleviate inefficiencies across workflows for their biomanufacturing customers. From scale-up and regulatory considerations to commercialization, issues of reliable, safe sourcing and efficient downstream chromatography processes provide opportunities for suppliers to alleviate pain points in the workflows that produce both traditional and emerging drug substances.
Provision of reliably, globally sourced cGMP process materials
As high costs and low supply cause delays for biologics manufacturers, raw material suppliers must step in to help fill gaps. A globally integrated supply chain with built-in redundancies is the best way to ensure that slowdowns in the receipt of necessary materials are kept to a minimum. A close relationship between suppliers and biologics manufacturers will be crucial to the efficacy of this solution, as it will be necessary for suppliers to continually assess the evolving needs of their customers. Likewise, this relationship would allow drug manufacturers to work with their suppliers to maintain a reliable supply of necessary materials, including those like pDNA that are difficult to secure in large quantities, and secure new nonstandard raw materials.
The supplier-manufacturer relationship will also allow the development of a shared characterization of critical quality attributes for raw materials. Ultimately, a consistent supply of high-quality materials will not only reduce the slowdowns caused by delays in the receipt of raw materials but also improve product consistency entering the downstream phase.
Downstream chromatography
There are two primary ways in which the supplier-manufacturer relationship can drive improvements in downstream chromatography processes. First, because chromatographic processes are sensitive to buffer conditions, suppliers can suggest workflow improvements and supply the necessary raw materials in a convenient form. A complete, curated portfolio for downstream chromatography process needs ensures that suppliers can deliver buffers, resins and other materials that meet the specific needs of a customer’s process and product.
Suppliers can also support customers in the selection of an appropriate resin that will help to alleviate many of the pain points discussed earlier. Though chromatography resins have existed for decades, they are still being improved upon through the use of additives to achieve specific conditions or the development of new ligands that have an increased dynamic binding capacity (DBC).
For example, protein A resins are the most widely used resins in the first purification step of mAbs. This is largely due to protein A resin’s ease of implementation, high specificity and strong regulatory track record. Despite the resin’s advantages, the cost of the protein A capture step is significant and leaves room for improvement. While the optimization of buffer preparation goes a long way toward improving the performance of a protein A resin, resins themselves can be enhanced to reduce time and cost while increasing yield.
A resin with a higher DBC improves the productivity of the capture step without necessitating a larger equipment footprint, saving manufacturers money on equipment. The increased efficiency resulting from a higher DBC also means that fewer cycles are required to capture the desired material, meaning that overall downstream processing time is reduced. This leads to lower operational risk, decreased labor and consumables cost, and potentially a smaller equipment footprint as lower buffer consumption enables smaller buffer tank sizes.
This example demonstrates the cost savings and reduced barriers to scale-up and time-to-market that result from more efficient downstream chromatography processes. Similar innovations are taking place with other chromatographic resins and technologies, and optimization in this phase will lead to improved downstream efficiency overall. While protein A resins are used in affinity chromatography for mAbs, other resins and methods are used for other modalities. For example, high-pressure liquid chromatography (HPLC) can be improved for increased purification efficiency of mRNA products.
Transformation through collaboration
While it’s a remarkable time for the biopharma industry, it is important to acknowledge that emerging opportunities come with new challenges. The evolution of new modalities and therapeutic areas — from mRNA vaccines against COVID-19 to cell and gene therapies for challenging diseases — has led to an emphasis on biomanufacturing innovations because products like cell and gene therapies require efficiency and flexibility in the supply chain as well as in manufacturing processes. The explosiveness of this growth means that efficiency and flexibility must be developed rapidly and alongside new and nonstandard processes and materials.
A strong supplier-manufacturer relationship can ensure that biologics manufacturers have what they need when they need it, even as those needs are rapidly evolving. Both parties must make an effort to create a fruitful relationship, however, as suppliers are limited by their knowledge of their customers’ needs. By involving suppliers early in a process, manufacturers can reduce cost and speed-to-market, leading to a collaborative biopharma ecosystem that may help safely and quickly deliver therapies to the people who need them. The future of biopharma is a complex and rapidly evolving topic, but it is clear that collaboration is set to transform the industry.