Shortening the equipment qualification path
In any major pharma manufacturing project, whether it is a greenfield project or a modernization to replace obsolete equipment, validation is often on the critical path. Therefore, project teams employ many strategies to compress the equipment validation process because it is a major contributor to costs and potential delays.
Ultimately, the total time and cost of the full validation process can be five to ten times what was spent on the equipment — a controller that costs $5,000 to purchase can easily cost more than $25,000 to validate.
Modern technologies and methodologies have made it easier than ever to compress the equipment validation timeline to save time and money. As plants continue their digital transformation journeys, advanced automation technologies, such as a modern distributed control system, and standards such as ASTM E2500, provide many opportunities to streamline the three core stages of the equipment validation process: installation qualification, operational qualification and performance qualification.
Improving installation qualification
To ensure all a plant’s operations and products are safe and effective, every piece of equipment, from complex bioreactors to individual transmitters, must be installed correctly. Confirming installation in a traditional project requires referencing wiring diagrams and confirming the physical installation of each connection from the equipment through a patch panel and wiring cabinet, and eventually into the control system. In the largest facilities, this can easily mean confirming the correct installation of thousands of I/O points via a cumbersome process of verifying wiring diagrams and performing loop testing.
Not only must project teams keep track of the paperwork associated with individual designs, but they must also create the documentation to show how they verified and tested each connection to confirm it was performed correctly.
These traditional methods result in high project costs and make it harder to implement changes as the project progresses. Even when changes are easy to physically implement, the time and costs associated with added documentation and testing to prove the correct installation for the change are often prohibitive.
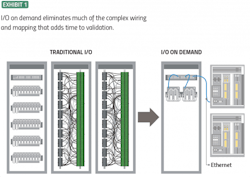
Advanced automation software and technologies can dramatically reduce the time and cost of installation qualification. Modern control systems provide far more flexibility and ease of use with I/O on-demand. These systems use interchangeable terminal blocks in the field, all connected to the control system via a single Ethernet cable. Once the I/O module is in place, it is automatically mapped to the control system. Field wiring of any signal type can be terminated on any I/O module, resulting in much less work, along with fewer contact points and associated potential failures.
I/O on demand simplifies wiring documentation by eliminating internal cabinet cross-wiring. Physical wires, wiring screws, panels, and even entire cabinets are often no longer needed, along with their associated test documents. Instead of spending hundreds of hours labeling wire terminations and verifying cabinet drawings, when project teams are ready, they can simply move to loop testing without extensive verification exercises. Teams eliminate documentation and testing by demonstrating that much of the previously required complex wiring and mapping no longer need to be performed (Exhibit 1).
Innovative control system manufacturers have also begun making independent skids easier to integrate into the control architecture. Advanced skid controllers feature native integration with technologies such as Module Type Package and native controller merging, eliminating the complex mapping necessary to connect the skid automation to the balance of the plant. Instead, all production data is collected in one system. Skids are automatically recognized by the control system without the need for extra effort or documentation to prove the integration works per design.
Improving operational qualification
When project teams create an operational unit, they must test all the operations and phases associated with the unit as part of the operational qualification stage of validation. In traditional projects, each unit instance must be validated independently, regardless of its similarity to other units in the plant.
However, most of the equipment in pharma facilities can be categorized into similar unit operations. For example, consider a new plant with three identical bioreactors, except for a difference in tag names. When the project team knows one of these units is configured properly, it should be able to quickly confirm the other two are as well. But when all units are considered independent entities, they must still be manually validated individually.
Modern, class-based control systems offer a faster, more streamlined approach to validation. Instead of treating each unit as an independent entity, class-based systems create a unit class containing all the operations associated with a unit. In the above example of the three identical bioreactors, all three can be collected in a single class.
With a class-based system, project teams can test once, after which they only need to test any minor differences among units. The off-the-shelf control system software verifies that everything maps, and the only remaining task is to validate the tags. For systems with duplicate equipment, class-based control can cut the validation time by at least half, and even if there are small differences among units, the project team can map those differences and test only the variations.
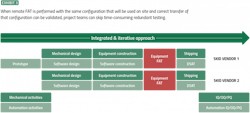
The time spent in both factory acceptance testing (FAT) and operational qualification also impacts both project timelines and costs. Traditionally, project teams would test automation equipment at the manufacturer’s site, but they would still need to fully test again when the equipment arrived at the plant (Exhibit 2).
Today, many project teams are reducing project timelinesby following ASTM E2500 lean validation guidelines. This lean validation approach leverages factory acceptance testing to meet some of the operational qualification requirements, eliminating duplicate work and shortening project timelines.
Remote FAT tools are another piece of the lean validation puzzle, enabling simultaneous remote testing of equipment to shorten the time to full installation and reduce validation steps once the equipment is installed. Using remote FAT, project teams test the actual equipment they will use in the plant while it is still at the manufacturer, generating documentation to show both the equipment and its configuration work per design. Using cloud-based FAT tools, those teams can remotely connect to the equipment and run tests using the actual system configuration. Once testing is complete and equipment is on-site, the team can use those same cloud tools to easily move the configuration from the development system into the production system.
The most advanced control systems document the configuration transfer in the change management system, making it possible to prove the code used successfully in FAT is the same code on the production system, eliminating the need to perform further qualification steps after installation. In addition, these same change management tools offer robust features to simplify troubleshooting in the validation process by creating more thorough audit trails, including the ability to roll back to previous configurations when changes cause problems.
The combination of these technologies and methodologies results in compressed calendar time (Exhibit 3).
Improving performance qualification
Performance qualification is the final stage of equipment validation, and it helps the project team prove the plant’s processes run properly within an acceptable range of variables and prove the product meets required specifications. Often, the greatest barrier to rapid performance qualification — and, accordingly, a barrier to late-stage changes — is finding ways to collect all necessary data.
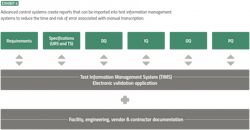
If process, cleaning and sterilization data are all stored in separate siloed systems, the validation team must spend time finding that data, standardizing it into a usable format, and manually documenting performance qualification test results. Each of these steps takes time, and each is subject to human error that might result in missed data or inaccurate transcription.
With a modern control system, all process data is centralized for fast collection. Qualification teams can run reports directly from the control system to rapidly pull together all the information they need. Moreover, advanced control systems can automatically generate test results that can be directly imported into test information management systems, eliminating the need to spend time tracking down and transcribing data (Exhibit 4).
Equipment lead times have been challenging in the last few years, so project teams are using simulation to test offline and train operators before equipment is even available. Simulations allow engineers to design and test configurations as though they were working with real equipment, helping to keep testing and training off the critical path.
Equipment delivery is often the critical path of a project, and expediting it is costly. Moreover, single-use items, bags, tubes and other disposable elements all add to the cost of qualification testing. By working the flaws out of processes in a simulation, and by training operators on that same simulation, qualification teams can reduce waste, human error and the number of required tests.
Not only are qualification teams expected to prove that processes work, but more and more frequently, they are being asked to demonstrate that no changes have been made to the process since validation, to ensure everything operates exactly as reported. Control systems with advanced change tracking and reporting tools provide audit trails with performance data to better demonstrate change management, and to validate current processes are identical to tested processes.
Better, faster validation
Performance qualification is the critical path for many projects, so each step taken to shorten equipment validation provides significant value because the sooner validation is complete, the sooner the plant is up and running, and the sooner patients are receiving crucial treatments.
Automation methods and technologies centered on a robust control system put the building blocks in place to help prove equipment is installed correctly the first time, that it operates as expected, and that all processes perform to specification, enabling higher quality equipment validations and improving lifesaving drug products’ speed-to-market.