Applying deep learning AI in toxicologic pathology
The newest developments in digital pathology, specifically the use of deep learning convolutional neural networks (CNNs) for digital image analysis (DIA) of scanned whole slide images (WSIs), promise to drive transformational advances in the 20’s and beyond for a critical scientific field, toxicologic pathology.1 Toxicologic pathologists support the safety evaluation of chemicals, pharmaceuticals, and medical devices that are designed to improve human and animal health, protect workers, and ensure the safety of water and food supplies.2 These specialized scientists author detailed safety assessment reports using disease and toxicity models.3 In these models, the pathologist evaluates changes in small samples of tissues placed on microscopic slides as well as data obtained from blood and urine. The toxicologic pathologist’s report is included in a detailed data package that drives progress in research and development and contributes to governmental regulation within the medical and chemical industries.
In 2019, toxicologic pathologists at Charles River Laboratories (CRL), one of the largest companies working in this research area, evaluated over 4 million microscope slides in support of these safety assessment studies. The evaluation of these slides alone required approximately 70,000 person hours for CRL and were on the critical path for support of client development efforts. When findings are identified, consistent and reproducible diagnostic scoring is considered best practice and requires additional effort including peer to peer consultation or a formal review from a second pathologist.3 Complicated changes within tissues may demand multiple diagnoses for completeness. The integration of the diagnostic data into an interpretive report is critical to support quality across the development life time of the tested material and is a high priority for the industry.4,5 Finally, because of improvements in laboratory in vitro, ex vivo, and in silico technology, early toxicology screening has reduced the number of test agent related changes and, in effect, a large proportion of these tissue slides are normal or only have spontaneous changes expected for the model system.
The rise of digital image analysis
Digital image analysis has great potential to assist the toxicologic pathologist with this daily workflow. While DIA is not new to the medical and toxicologic pathology industry, technical improvements in computer hardware and software performance, graphics technology, and memory storage capabilities are rapidly advancing this field.1 Toxicologic pathologists are now positioned to take advantage of these technical improvements and incorporate AI-CNNs into our art. Early investigations into the utility of AI are underway that demonstrate the potential for the technology to drive quality, efficiency and innovation in toxicologic pathology diagnostics. Three examples are briefly highlighted here in an effort to represent the potential impact of AI-CNN in toxicologic pathology.
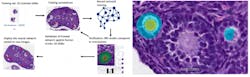
Evaluating potential toxicity that may result in decreased fertility is an important component of the development testing funnel. In one assay required by regulators, the toxicologic pathologist assesses a test agent’s effect on the ovary by enumerating the number of primordial and primary follicles. This painstaking work is both time consuming and prone to intra- and inter-observer variability. To improve the counting process, additional procedures have been instituted including immunohistochemistry and standard image analysis to facilitate the counting.6 AI-CNNs have now been developed by toxicologic pathologists and others to classify and count these structures in standard hematoxylin and eosin-stained slides in an automated fashion.7,8 (Figure 1)
Figure 1: Workflow for training and testing of AI-CNN (Aiforia Create) for ovarian follicle counting; Figure 1B: AI-CNN identifying and counting primary follicle (left) but not counting a potential primary follicle (right) that did not meet requirements (nucleus not clearly present)
By simply digitizing the microscopic slide, the AI-CNN algorithm in seconds can complete what takes several minutes by a trained observer. The algorithm can be cross-trained to evaluate the ovary from different toxicology models quickly or adapted to answer specific investigative questions that the laboratory may have concerning the time course or mechanism of toxicity. The AI-CNN algorithm can be placed in the “cloud” and the same algorithm be used by multiple pathologists globally.9 This simplifies the need for software installations and ensures that laboratories may be consistent. Similar approaches have been developed for the testes as well and for quantifying mitoses in various tissues. AI-CNNs promise to automate these types of redundant tasks for the toxicologic pathologist.
Testing for carcinogenicity
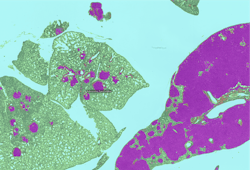
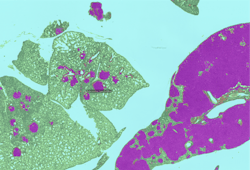
Carcinogenicity toxicology studies are one of the most complicated testing paradigms used in the safety assessment industry. Tissue evaluation involves upwards of 50,000 slides per study by the toxicologic pathologist. The primary purpose of these studies is to characterize any proliferative changes that may indicate that the test agent increases the risk for cancer in humans or animals. Sensitivity and specificity are important in these studies as is the toxicologic pathologist’s assessment of the relevance of any finding to human or animal health.10 AI-CNNs at CRL were recently developed to identify proliferative changes in the lung in a common model system used for carcinogenicity. (Figure 2)
Figure 2: AI-CNN prediction (Deciphex Patholytix AI) of lung adenomas in urethane-treated TgRasH2 mice
The toxicologic pathologist trained the AI-CNN to recognize two different important classes of proliferative lesions expected with the treatment administered. After training, the AI-CNN algorithm identified lesion hot spots in a test set at 100 percent concordance with a group of six toxicologic pathologists.11 This screening approach promises to accelerate the workflow for the toxicologic pathologist in carcinogenicity studies and is being investigated in additional tissue types. The AI-CNN could prepare a visual data set that is delivered to the pathologist with the study slides. The pathologist would use the screening data to support an efficient and targeted approach to analysis and provide study sponsors with an earlier indication of potential toxicity. We expect the AI-CNN will also accelerate the entire slide review process. As the pathologist identifies novel lesion patterns in the tissues, additional training could be provided to the AI-CNN to increase the breadth of the algorithm’s ability to not only identify the abnormal but also suggest standardized diagnostic terminology and when appropriate a severity grade.
Certain microscopic changes in toxicology models are encountered commonly by the toxicologic pathologist. This is especially the case for the liver which is a frequent target because of its metabolic machinery and role in the detoxification after oral challenge. Liver enzymes in the cytochrome P450 family may be induced by the test agent in toxicology models resulting in increased liver cell (hepatocytes) size and organ weight. Hepatocyte enlargement may be challenging to discern by the human eye especially when it is a diffuse change.12 An AI-CNN algorithm was developed to examine hepatocyte size differences between treated and control groups. The algorithm classified hepatocytes and then used area measurements for comparison. The pathologist received both visual and quantitative data (areas) to provide decision support in threshold setting and grading. The preliminary visual and area data was highly correlative to liver organ weights and pathologists scores.
Figure 3: AI-CNN (Visiopharm AI) identifies hepatocytes and postprocessing calculates hepatocellular areas for decision support in hepatocellular hypertrophy diagnoses
The AI-CNN is also now being trained to recognize changes in hepatocytes based on the portion of the hepatic lobule affected, an important consideration for the pathologist. If successful, a liver AI-CNN may provide significant diagnostic support to the toxicologic pathologist for the diagnosis of this common change. We are also examining other tissues (e.g. adrenal gland, bone physis, and thyroid gland) where diffuse changes in cell size or number may occur and are measured qualitatively by the toxicologic pathologist. It is anticipated that a menu of AI-CNNs could be developed that a toxicologic pathologist could apply when diagnostic support facilitates data generation and interpretation. The AI-CNN becomes a consultant for the toxicologic pathologist and supports their workflow and day to day consistency in their data generation.
The future of toxicologic pathology
The examples discussed above all require digital whole slide scanning of slides. However, it is not uncommon for toxicologic pathologists to work remotely and to not have immediate access to digital scanning capabilities. With that in mind, methods are under development to allow the toxicologic pathologist to evaluate microscopic slides using standard image analysis and AI-CNNs that are directly integrated into through-the-light microscope hardware.13 Augmented reality methods would not require digital slide scanning and may provide real time decision support by a DIA or AI-CNN algorithm directly through the light microscope oculars.
With the commercial availability of user-friendly CNNs, scientists and toxicologic pathologists from all over the globe are collaborating with computer scientists focused on AI-CNN development and application. The momentum of this technology in medical and toxicologic pathology is exciting and fueling a hub of innovation within the field. AI-CNN’s have potential to provide decision support for Toxicologic Pathologists and drive both workflow efficiency and more objective, consistent quantification into the qualitative and descriptive science of pathology. Toxicologic pathology is entering a digital renaissance in pathology sciences and the 20’s will be a key decade for further research and testing.
References
1Turner OC, et al. Society of Toxicologic Pathology Digital Pathology and Image Analysis Special Interest Group Article*: Opinion on the Application of Artificial Intelligence and Machine Learning to Digital Toxicologic Pathology (2019). Toxicol Pathol DOI: 10.1177/0192623319881401
2Ochoa R. In: Fundamentals of Toxicologic Pathology, 3rd ed (2018), p. 105
3Morton D, et al.Recommendations for Pathology Peer Review. Toxicol Pathol https://doi.org/10.1177/0192623310383991
4Morton D, et al. Best Practices for Reporting Pathology Interpretations within GLP Toxicology Studies (2006). Toxicol Pathol https://doi.org/10.1080/01926230601034624
5Mann PC, et al. International Harmonization of Toxicologic Pathology Nomenclature: An Overview and Review of Basic Principles. (2012) Toxicol Pathol https://doi.org/10.1177/0192623312438738
6Picut C, et al. Ovarian Follicle Counts Using Proliferating Cell Nuclear Antigen (PCNA) and Semi-Automated Image Analysis in Rats. (2008) DOI: 10.1177/0192623308317428
7 Sonigo C, et al. High-throughput ovarian follicle counting by an innovative deep learning approach. (2018). Scientific Reports. DOI:10.1038/s41598-018-31883-8
8Rudmann DG, et al. and Marxfield H, et al. European Society of Toxicologic Pathology, Koln, Germany, September 2019.
9https://www.aiforia.com/blog/crl-pathologists-use-case/
10Kerlin R, et al. Scientific and Regulatory Policy Committee: Recommended (“Best”) Practices for Determining, Communicating, and Using Adverse Effect Data from Nonclinical Studies. (2015). Toxicol Pathol. https://doi.org/10.1177/0192623315623265
11 Rudmann DG, et al. American College of Veterinary Medicine, San Antonio, Texas, November 2019.
12 Aeffner F, et al. The Gold Standard Paradox in Digital Image Analysis: Manual Versus Automated Scoring as Ground Truth. (2017). Arch Path Lab Med. DOI: 10.5858/arpa.2016-0386-RA
13 https://www.augmentiqs.com/augmented-reality-microscope/