Pharmaceutical manufacturers are adjusting to a new set of realities, from the influx of new gene- and cell-based therapies, to the expectation that therapies will be more readily available at points of care, including patients’ front doors. While that progress should be celebrated, it also poses new challenges for traditionally rigid and unreliable cold chain processes. Additionally, it underscores the urgent need for transport to become highly flexible and infinitely more dependable than current systems.
Ultimately, manufacturers have two choices when it comes to achieving greater cold chain reliability and flexibility: make or buy. Choosing to make a cold chain system can offer companies a sense of control and flexibility over the process. But it also requires significant investments in money, time and resources to develop, engineer and operate their own state-of-the-art cold-chain systems that can handle the intricacies of a thriving and constantly changing global marketplace.
On the flip side, choosing to buy into a holistic solution with a provider that specializes in cold chain logistics can create an easier way to guarantee that drugs will remain safe and effective as they’re transported to patients around the globe.
A Changing Industry
The pharmaceutical industry loses tens of billions of dollars as a result of temperature excursions, making transport one of the weakest links in many manufacturers’ supply chains.1 Despite these underwhelming statistics, the industry and its regulators can and will continue to demand zero excursions, and the technology and infrastructure are certainly there to support that.
At present, millions of shipments per year arrive at their end destination out of spec for temperature. That’s unacceptable, since excursions are likely to affect drug efficacy and patient safety, yet not entirely surprising considering the number of people and imperfect processes that can “touch” a product during transport. With advances in life sciences R&D, more manufacturers are producing temperature-sensitive, high-value biologics, and this means the risk of costly, life-threating temperature excursions will only grow.
In the last few years alone, more than half of the top 50 global drug products required cold chain (temperature controlled) handling. By 2022, the number is projected to be greater than 60 percent, with total sales approaching $150 billion. Within 10 years, it’s estimated that temperature-sensitive drugs will account for more sales than non-temperature-sensitive drugs.
For many manufacturers, a truly connected cold chain remains inconceivable. They may make incremental progress, from using new packaging to more efficient transport and sensors, but many still struggle for consistent, reliable performance. Regulatory requirements from the U.S. Food and Drug Administration, European Commission and other entities worldwide are certainly an important driver, but the pressure is still on the manufacturers to ensure that their products meet or exceed quality standards, are safe, and work as intended when they reach patients.
From cancer treatment to flu vaccines, exposing drugs to temperatures outside of recommended specifications during transport can have lasting and damaging effects on manufacturers. At a minimum, they may be subject to violations and fines from the FDA, and are forced to absorb the costs of spoiled or damaged products, affecting the company’s bottom line and drawing the ire of stakeholders. Brand reputation can also be severely or permanently damaged if manufacturers cannot deliver safe products. In a worst case scenario, compromised drugs make their way to patients.
Until recently, manufacturers have had little choice but to manage the entire process themselves, from pack-out (including everything from boxes to dry ice), to shipping-lane decisions and data collection. This requires hefty upfront and ongoing investments of time and resources. And for most manufacturers, managing a complex cold chain falls outside of their core competencies, distracting them from their primary mission: savings lives.
By outsourcing complex cold chain functions to experts, manufacturers can avoid making investments in infrastructure and technology that quickly becomes outdated. Those investments are better left to third parties who are constantly refining cold chain technology and processes and can promise zero excursions and 100 percent accountability.
Defining Cold-Chain-as-a-Service
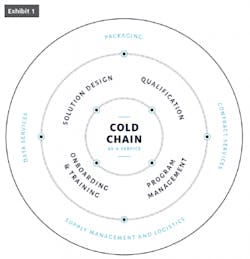
A cold chain isn’t one thing. It’s a combination of products and services that include vital links of packaging, storage, transportation and monitoring, all working in unison to ensure quality and consistency. It’s complex but necessary to accommodate product that has an equally complex composition which can be rendered dangerous or ineffective by even the slightest variation in temperature. What’s needed for an uninterrupted cold chain is seamless integration that ensures reliability and flexibility without sacrificing simplicity.
When organized in a highly integrated fashion, including easy-to-pack, reliable containers; shipping management, data analysis and more, this unbroken chain is aptly called cold-chain-as-a-service (CCaaS). Like its corollary software-as-a-service (SaaS), CCaaS is predicated on maintaining quality, safety and efficacy from development to end use. Best practices and technological innovation are perpetual and expected, accounting for the newest regulatory requirements and introduction of new drugs with even tighter cold-chain handling tolerances.
Today, as much as five percent of gross revenues are spent on cold chain logistics, according to data from the 2018 Biopharma Cold Chain Sourcebook. Given this outlay, it’s no wonder manufacturers are looking for more holistic solutions. For those seeking to implement CcaaS practices, the exercise must start with an end-to-end solution design; supply management and logistics, including all pre-pack-out activities (preconditioning, kitting, etc.); just-in-time delivery; data services, including payload and package data captured on-site and in transit; and program management, including inspection, repair, reconditioning and re-kitting. Manufacturers must also factor in the cost of containers that cannot be reused, packing material and other waste from the shipping process.
Benefits Abound
Because of the high cost and extensive time required to design and manage their own efficient, flexible, reliable and sustainable cold chain, drugmakers are increasingly turning to outside vendors to handle critical packaging and shipping needs. Another major driver for manufacturers is the increasing adoption of custom-designed solutions and just-in-time deliveries that require smaller packaging and inventory footprints. This can free up existing valuable warehouse space for better, more profitable (or less costly) use.
By outsourcing cold chain logistics, biopharma manufacturers can more easily adapt to national and global market demands and navigate a web of regulations that can vary greatly from state-to-state and country-to-country, resulting in increased scalability and standardization once a drug is approved. This is critical when ramping up production in response to a global health emergency such as an Ebola outbreak in a populous region of Africa, when the rapid availability of a reliable, effective vaccine may be the best defense against the spread of the highly contagious and deadly disease.
While greater vigilance and visibility into the packing and shipping process can help ensure temperature-sensitive therapies maintain their efficacy and are safe, there’s another problem costing the industry millions of dollars annually that cold chain service providers can help address: Waste.
Standard prequalified polystyrene (EPS) and polyurethane (PUR) insulated containers aren’t as efficient as technology-enabled, reusable solutions from cold-chain service providers. The outdated vessels prevent drugmakers from shipping with purpose and are often overpacked with insulation materials because of uncertainty about weather, time and other factors, which contributes billions of tons of waste to landfills globally. This is a shame, since available solutions can decrease a company’s landfill contributions by as much as 99 percent.
In some cases, reuse programs are fully managed by the vendor. With this approach, the containers arrive to the client ready for use with no need for on-site preconditioning, resulting in improved efficiency. Once the customer loads in and ships its product and delivery to the end destination is complete, the container is recovered, inspected, repaired if necessary, and then reconditioned and returned to the client for use.
Getting Started
Any cold chain solution must be comprehensive, holistic and capable of ensuring zero excursions. Any compromise or concession casts doubt on the system as a whole. Many prefer outsourcing their cold chain because the complexity and technical skillsets required puts them at significant risk if they go it alone. The idea of shipping product with zero excursions without increasing costs — and maybe even lowering them — is becoming a compelling value proposition for many.
In either case, a recommendation for manufacturers is to start with a checklist of must-haves. As mentioned above, the following is a starting point — or at least what should be expected of any external partner:
- Customized solution design
- Supply management and logistics, including all pre-pack-out (preconditioning, kitting, etc.)
- Just-in-time delivery capability
- Data services, including payload and package data captured on-site and in transit
- Program management, including inspection, repair, recondition and re-kitting
In addition, the packaging technology used for transport must be proven and state-of-the-art. While it’s not just about the packaging, a poorly designed container simply cannot support the reliability, flexibly, simplicity and sustainability required for the age of biopharmaceuticals and other temperature-sensitive drugs. In the end, it’s all about zero excursions, and whatever solution you choose, make sure you’ve checked all — and have all — the right boxes.
References
IQVIA Institute for Human Data Science, formerly The IMS Institute