Nearly two decades have elapsed since stem cells were debuted on the new medical therapies stage with much fanfare. The first act of the highly touted show starred stem cells obtained from human embryos as magical new cell medicines with potential to remedy a panorama of chronic debilitating diseases and ailments for which no cures or even palliative treatments existed. Human embryonic stem cells (hESCs) also had the important practical attribute of proliferation properties that were suitable for future mass manufacturing. Good manufacturing potential was an important quality for enticing pharmaceutical companies to consider entering the arena of cell therapy development, much in the way that they had entered the new arena of biologics therapy development, 25 years earlier, after technologies for the mass production of biologic drugs were established. A similar confluence of drug development elements appears under way now for CAR-T cancer immunotherapy. Therapeutic effect and manufacturing capability, these are two essential elements that a new therapeutic discipline needs to attract the interest and investment of big pharma.
So far, in the case of stem cell therapy, the discipline has experienced disastrous mismatches between the ability to manufacture different types of stem cells and their respective therapeutic efficacy. These recognized, but greatly underestimated, challenges have kept the pharmaceutical industry largely on the sidelines of stem cell medicine. This state of affairs is a major impediment to progress in stem cell regenerative medicine by depriving the emerging new medical discipline the acceleration that full engagement of the pharmaceutical industry could bring.GOOD MANUFACTURING POTENTIAL, POOR THERAPEUTIC POTENTIAL
Much to the chagrin of their promoters, very soon after hESCs took the stage, the curtain came crashing down. The fall is most often blamed on outbreaks of moral and ethical objections to the first step in the production process for hESCs. The source human embryos were destroyed as a result of the extraction of the precursor cells used to produce hESCs. Many considered this unavoidable processing step sufficiently unsavory to warrant prohibiting the production and use of hESCs for research or development of new therapies.
In reality, there were many other factors that would have brought the curtain down as well, even if the moral and ethical concerns could have been appeased. The truth of this statement is evident from the historical course of induced pluripotent stem cells (iPSCs). Similar to hESCs, iPSCs have the ability to convert into many different types of body cells. This property, called pluripotency, has been the main selling point for both hESCs and iPSCs. However, they differ in their origin in a very important manner. The production of iPSCs does not require human embryos, and therefore is free of moral and ethical concerns.
Like hESCs, iPSCs have shown potential for manufacturing success. Several biotechnology companies now market manufactured iPSCs for research purposes. Unfortunately, this manufacturing progress has not been matched with therapeutic progress or potential. Like hESCs that inspired their development, iPSCs have many biological and technical shortcomings that pretty much eliminate them from having a future substantial impact in stem cell medicine.
First and foremost, like hESCs, iPSCs are potentially very dangerous, because they form tumors when injected into the mature tissues of animals. Second, it is now recognized that these pluripotent stem cell types do not have the previously widely pronounced magical property of “being able to make every type of cell in the human body.” So far, they have proven quite restricted in their ability to produce mature human tissues as originally envisioned. Though they can be induced to convert into some types of tissue cells with greater maturity, the degree of maturation is limited to fetal cell-like properties. The fetal-like cells will have inadequacies for use in both cell therapy and drug development for disorders in children and adults. In addition to inadequacies due to immature development, mature cells produced from pluripotent stem cells are also at high risk for functional deficiencies caused by gene defects that pluripotent stem cells are known to harbor at high rates.
Finally, though less noted, even if pluripotent stem cells did not form tumors, they would still be deficient for producing long-lasting cures in children and adults. They lack an essential property required to maintain, repair, and restore mature human tissues. That property, called asymmetric self-renewal, is the unique province of another type of stem cells, adult tissue stem cells (TSCs).
GOOD THERAPEUTIC POTENTIAL, POOR MANUFACTURING POTENTIAL
Adult tissue stem cells are rare cells found in all mature tissues in children and adults. They are responsible for the continuous renewal and repair of mature tissue cells. Asymmetric self-renewal by TSCs is characterized by asymmetric stem cell divisions that produce both stem cells and committed cells simultaneously. Stem cells can divide indefinitely, but their tissue fraction remains low and constant because of the format of the asymmetric self-renewal tissue system. The committed cells produced by asymmetric stem cell divisions undergo many additional divisions to produce the mature cells of the tissue. However, all of the last cells produced in a committed lineage undergo a permanent arrest from division when they reach final maturity. When old mature cells expire, next-in-lineage maturing cells replace them. As the base cell for this cellular replenishment system, stem cells keep renewing mature tissue cells while their own number is maintained at a very low fraction of total cells. There are many developmental and evolutionary advantages ascribed to this universal, unique property of TSCs in mammalian organs and tissues. In addition, under conditions of injury, TSCs can divide symmetrically, producing only stem cells transiently for the purpose of making new tissue units for the repair of tissue cell defects.
Given their natural roles in healthy tissue function, repair, and disease (e.g., cancer development), adult TSCs are an obvious choice for development of new stem cell medicines. Yet, the pharmaceutical industry has made only limited forays into stem cell therapy or even development of new medicines based on stem cell effects. There are two long-standing technical barriers in stem cell biology and biomedicine that account for this current condition. First, expansion of adult TSCs in culture has proven essentially impossible. Second, there is the long-standing unmet need for a means to count adult TSCs specifically. The recent accomplishment of a method to breach the second barrier may very well provide the means to vanquish the first as well, opening the door to more pharmaceutical investment in stem cell medicine.
The nature of the adult TSC expansion problem becomes readily apparent after even a cursory look at the kinds of stem cells that are the focus of current therapy trials, whether in FDA-authorized clinical studies or in private stem cell clinics. Two main types of treatment preparations are pervasive. Treatments prepared to contain stem cells for blood, called hematopoietic stem cells (HSCs); or treatments prepared to contain mesenchymal stem cells (MSCs). HSCs are harvested from umbilical cord blood, bone marrow, and cell fractions mobilized into the bloodstream by treatment with specific growth factors. MSCs are collected from a variety of tissues, including bone marrow, umbilical cord, fat tissue, and amniotic fluid.
What these treatment preparations share in common that motivates their use are relatively accessible, large numbers of cells with high rates of proliferation. In retrospect, it is understandable how stem cell biotechnology companies and clinics have been easily lured into this very deceptive quicksand. HSCs are the gold standard for efficacious stem cell transplantation medicine when it comes to stem cell replacement treatments. Yet, even in this approved clinical setting, it is well established that attempts to increase HSC number by culturing invariably results in a significant loss of their efficacy.
Now, most new stem cell transplant therapies — even those using HSC preparations — are not designed for replacement of homologous stem cells. Instead, most advancing treatments evaluate whether injected stem cell-containing preparations have healing effects in damaged and ailing tissues in general, whether or not the treating stem cell type is from the treated tissue type. Unfortunately, the lessons learned in HSC transplantation medicine have not been well considered in these new heterologous MSC transplantation trials, which are the major fraction of new studies.
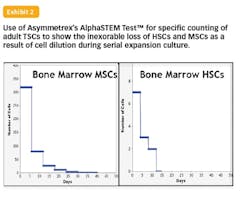
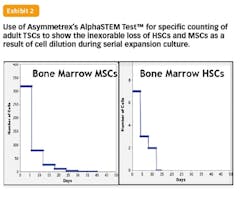
So, the stem cell medical field is now mired with mounting numbers of unsuccessful trials, many which were conducted with expanded MSC populations. Some issues are quite clear. By the measure of the ability to produce tissue-specific mature cell types, all reported manufacturing processes using expansion culture are troubled by significant losses of stem cell potency. Yet, other crucial issues are quite unclear. The cellular basis for the universal loss of adult TSC potency with expansion culture is still a mysterious bugaboo for many in the field of stem cell medicine in general. For the pharmaceutical industry to develop more interest in investing its resources, not only must the nature of this vexing problem be defined, but a practical solution for it must also be developed.SPECIFIC STEM CELL COUNTING
Given the many important functions of adult TSCs in health and disease, it is an irony that so little of current drug development focuses on stem cells as the basis for discovery and development of new and improved medicines. This surprising situation exists in the pharmaceutical industry because (prior to recent advances) there has been no means to count adult TSCs specifically.
This lacking has persisted because of the long-standing difficulty in defining biomarkers that specifically identify TSCs, but not their more abundant progeny, lineage-committed progenitor cells. This problem compromises the use of all previously described “stem cell biomarkers,” which has been somewhat of a misnomer. For example, well known “stem cell biomarkers” like CD34, CD133, and CD90, which are among the best-described biomarkers for HSCs as well as stem cells in several other tissues, also are expressed by committed progenitor cells, which are produced by the asymmetric self-renewing divisions of HSCs. Because committed progenitor cells are always significantly more numerous than stem cells, both in tissues and in isolated stem cell-enriched tissue cell preparations, they preclude any chance for quantification of specific TSC number.
The inability to count TSCs has limited stem cell-focused drug development efforts in two ways. First, in the case of interest in identifying stem cell-activating drugs, it is difficult to develop effective efficacy assays without a means to quantify TSC number. The second adverse impact is general for development of all classes of drugs. Drugs that are stem cell-toxic cause chronic organ failure. Chronic organ failure is estimated to account for roughly half of drug failures due to safety in Phase II and Phase III clinical trials. Currently, such unsafe drug candidates are not detected until either animal studies or patient studies in Phase II and Phase III. The ability to count TSCs would allow detection of the stem cell toxicity of such unsafe drug candidates much earlier and with much less expense in human cell culture tests. For pharmaceutical companies with billion dollar drug development budgets, the ability to identify stem cell-toxic drugs before more expensive animal studies and clinical trials could save hundreds of millions of dollars each year.
To the main point of this perspective, the new technology for specific TSC counting could also enable the right match of manufacturing and therapeutic potential needed to attract the full engagement of the pharmaceutical industry in the development of new stem cell therapies. The ability to count adult TSCs allowed Asymmetrex to quantitatively confirm a 16 year-old hypothesis formulated by the author to account for the loss of adult TSC potency in expansion cultures. The basic explanation is that loss of potency is not due to changes in TSC function. Instead, the potency loss mirrors the rapid loss of TSCs to dilution among accumulating committed cells with time as a result of asymmetric self-renewal in serial cultures. So far, all examined sources of TSCs, including human HSCs and human MSCs, have shown this mechanism of loss that was predicted to be universal for all types of adult TSCs.
Asymmetrex has also developed potential solutions for the TSC expansion problem. Inspired by the first report of the TSC dilution explanation in 2001, the company has developed several patented technologies that reverse the asymmetric self-renewal of TSCs as a means to induce their exponential expansion. Now with a specific counting tool in hand, it should be possible to further optimize these novel expansion technologies. Not only is it now possible to monitor the production of adult TSCs, but it is also possible to determine their treatment dose for the first time. These new advances put stem cell medicine more in line with the benchmarks that can attract Big Pharma and, perhaps, eventually their full engagement in stem cell therapeutics.
ABOUT THE AUTHOR
James L. Sherley, M.D., Ph.D. is the founder and director of Asymmetrex, LLC, a Massachusetts biotechnology company that develops innovative tools for advancing stem cell medicine.