Dealing with Pharma's Digital Demons
Every industry has its demons. The pharmaceutical industry’s struggles — its aversion to risk, fear of regulatory backlash, the siloed nature of its organizational structure — are well documented.
But today’s developing environment — coined the Fourth Industrial Revolution and characterized by rapidly evolving and disruptive advances in technology — has created a new and intimidating landscape. According to the World Economic Forum, this current revolution is “reshaping industries, blurring geographical boundaries, challenging existing regulatory frameworks, and even redefining what it means to be human.”¹ In this new world, tools such as automation, IIoT, cloud-computing and data analytics offer all industries the potential for digital transformation — if they can properly understand and implement them.
Pharmaceutical Manufacturing’s second annual Smart Pharma survey separately asked drug manufacturers and equipment and services vendors* their thoughts on the pharma industry’s digital transformation progress.
Close analysis of the survey results confirms a lot of what we already know: The pharmaceutical industry has seen great advances in transformative technology, and it’s becoming increasingly evident that if properly harnessed, many of these innovations can give manufacturers an edge. But recent survey results closely mirror the responses from last year — and the concern is that this could mean progress is stalling.
In order to keep moving forward and propel itself into the next stage of digital transformation, pharma needs to confront some very real challenges.
THEY BELIEVE
The good news is that pharma believes in digital. Over 70 percent of surveyed pharma manufacturers see the big picture, understanding that in the long-run, a more automated pharma industry will improve productivity, quality, and efficiency — and cut costs. This year, the number of manufacturers who specifically indicated a connection between automation and cost-cutting jumped from 55 to 71 percent — perhaps a positive sign that manufacturers are starting to see actual financial benefits from successful digital initiatives.
Cultural buy-in, especially from a traditionally conservative industry, is also crucial for success. According to survey results, the industry is noting a rising comfort level with the new computing, control and communications technologies represented by the IIoT. Over 79 percent of pharma respondents noted an increase in automation-related comfort in the industry, while 83 percent of vendors (up 15 percent from last year) agreed that the pharma workforce’s comfort level is rising.
Encouragingly, when it comes to designing or upgrading manufacturing facilities, 87 percent of manufacturers and 91 percent of vendors said that digitalization is part of the discussion. Of this group, 36 percent of manufacturers said digitalization is actually the leading priority.
Survey answers support the assertion that the technological innovation needed for pharma to succeed on its digital transformation journey is available, and at increasingly affordable costs, too.
When ranking their concerns surrounding smart manufacturing plants, tech innovation — specifically the concern that “technology being offered to the pharma industry is not advanced enough” — was second to last on the list of manufacturers’ concerns and dead-last according to vendors. But perhaps vendors are warranted
in giving themselves a pat on the back, as it seems that overall, the industry is confident that the level of technology being offered is advanced enough to do the job.
These results are in consonance with the manufacturing sector in general, as well. At the recent Smart Industry 2018 Conference — an annual event geared toward accelerating the ongoing digital transformation of manufacturing — the exhibit hall was overflowing with innovative technologies. Yet during three days of presentations, the focus was not on technology, but instead, how to integrate and use these new tools to maximize benefits.
In pharma, there appears to be a debate as to who is responsible for continuing to advance this technology. According to survey results, pharma manufacturers are somewhat split between believing vendors should be proactively leading the charge, believing vendors should be reacting to the needs of pharma companies, or believing there should be collaboration between both parties. Vendors, however, are more decisive: Over 50 percent believe they should be leading the innovation charge, while a mere 12 percent feel they should be reacting to the demands of drug manufacturers.
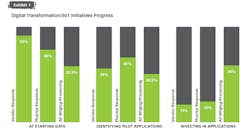
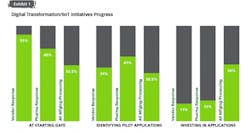
When asked to describe the pharma industry’s collective progress on digital transformation initiatives, the vast majority (87 percent of manufacturers and 89 percent of vendors) put the industry into one of two categories: at the starting gate, with focus on learning and exploration, or identifying early applications to pilot.
Pharma shares this reality with other industries as well. A recent collaboration between the World Economic Forum and McKinsey & Co.¹ found that more than 70 percent of industrial companies are still either at the start of their journeys or unable to go beyond the pilot stage. The latter stage is referred to as “pilot purgatory” — where technology is rolled out experimentally at reduced scale for an extended period due to the inability or unwillingness to deploy at a larger scale.
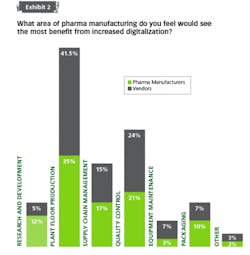
For pharma, when it comes to reaching the more advanced stage — identifying applications and making investments to match — it seems few manufacturers are willing to make the financial leap. Even at a basic level, when vendors were asked if pharma companies were looking to replace outdated equipment with improved robotics or controls automation, only 6 percent could decisively claim that most companies are actively updating.
In pharma, managers of legacy facilities are no strangers to addressing the problems associated with aging infrastructure, equipment and processes. The need to accommodate new, digital technology adds an additional layer of complexity. Fortunately, vendors are rising to the occasion, providing ways to upgrade technology without replacing existing machines. Devices such as serial servers and media converters can give existing equipment new connectivity options and the ability to communicate.
Regulatory hurdles slipped from the No. 1 concern to the second spot this year, for both manufacturers and vendors. With compliance being essential in pharma, it makes sense that lack of regulatory buy-in or understanding of new processes would continue to be a challenge. When vendors were asked directly what they thought was the top issue holding back their customers’ digital progress, fear of regulatory backlash (30 percent) and an unwillingness to pioneer unproven technology (27 percent) topped the list.
But as the industry progresses down its digital path, it’s important to involve regulators early and often. According to the BioPhorum Operations Group, (a cross-industry collaboration), pharma’s transition will require “diligence by industry to show regulators the benefits of the move, but still show the ability to maintain data integrity, demonstrate control and custody of operating software in an open environment, and validate the use of real-time release instruments and process models.”²
TIME TO LOOK UNDER THE BED
For a second survey year in row, pharma is finding itself at a familiar digital transformation crossroads: The industry understands the power of digital, has made a few pilot investments, but can’t continue its journey without fully investing.
And pharma isn’t alone. During this year’s Smart Industry Conference, Brian Irwin, automotive and industrial lead for IT consultancy giant, Accenture, pointed to research across multiple industries indicating that while 80 percent of industry executives expect both efficiency and growth from digital, only 13 percent are investing correspondingly. Across all industries, Irwin confirms, very few companies have made it to the phase of digitalization where they have found a way to see growth and profits from their digitalized business model.
Exactly how far away is pharma from truly reaping all the benefits of IIoT and the smart factory? According to our survey results, 64 percent of pharma manufacturers and 86 percent of vendors believe the industry is five to 10 years away. Yet, 27 percent of manufacturers still feel the industry is more than a decade away from reaching this goal, or will never actually get there — demonstrating that equivocation is still very real.
But this hesitation, according to McKinsey & Co., may not be the best course of action. McKinsey analysts (read our Data Management story ) describe small-scale digital initiatives and pilot investments as “self-limiting,” warning that they “will not generate the kind of dramatic results that leaders expect, potentially threatening future investments.” Instead, experts insist that in order to take full advantage of digital, companies need to think in comprehensive terms, by making more dramatic, systemic changes.
“Launching a ‘digital transformation’ can seem ambitious. But tentative half-measures will not capture the full potential from technology,” says McKinsey.
Those looking to keep up with frontrunners will need to put fears aside and confront their digital demons head-on, pushing themselves itself into the most rewarding stage of digital transformation.
>>>>Want to learn more about accelerating digital transformation in pharma? Don't miss our webinar.
*For the purposes of this survey, “vendors” are defined as companies who offer pharma processing equipment, lab equipment, controls or software, as well as consulting or related services. There were 70 vendor responses. “Pharma manufacturers” are defined as those who manufacture pharma or biopharma drugs, make APIs or excipients or offer contract services. There were 102 manufacturer responses.
REFERENCES
1. The Next Economic Growth Engine Scaling Fourth Industrial Revolution Technologies in Production. World Economic Forum, January 2018.
2. The Technology Roadmap for the Biopharmaceutical Manufacturing Industry. BioPhorum Operations Group, July 2017.
[javascriptSnippet]